import numpy as np
import pandas as pd
import matplotlib.pyplot as pl
from matplotlib import rcParams
import scanpy as sc
import scipy
import numpy as np
import matplotlib.pyplot as plt
import warnings
warnings.simplefilter(action="ignore", category=Warning)
# verbosity: errors (0), warnings (1), info (2), hints (3)
sc.settings.verbosity = 3
sc.settings.set_figure_params(dpi=100, frameon=False, figsize=(5, 5), facecolor='white', color_map = 'viridis_r') Code chunks run Python commands unless it starts with %%bash, in which case, those chunks run shell commands.
Partly following this PAGA tutorial with some modifications.
1 Loading libraries
2 Preparing data
In order to speed up the computations during the exercises, we will be using a subset of a bone marrow dataset (originally containing about 100K cells). The bone marrow is the source of adult immune cells, and contains virtually all differentiation stages of cell from the immune system which later circulate in the blood to all other organs.
If you have been using the Seurat, Bioconductor or Scanpy toolkits with your own data, you need to reach to the point where can find get:
- A dimensionality reduction where to perform the trajectory (for example: PCA, ICA, MNN, harmony, Diffusion Maps, UMAP)
- The cell clustering information (for example: from Louvain, k-means)
- A KNN/SNN graph (this is useful to inspect and sanity-check your trajectories)
In this case, all the data has been preprocessed with Seurat with standard pipelines. In addition there was some manual filtering done to remove clusters that are disconnected and cells that are hard to cluster, which can be seen in this script
The file trajectory_scanpy_filtered.h5ad was converted from the Seurat object using the SeuratDisk package. For more information on how it was done, have a look at the script: convert_to_h5ad.R in the github repo.
You can download the data with the commands:
import os
import subprocess
# download pre-computed data if missing or long compute
fetch_data = True
# url for source and intermediate data
path_data = "https://nextcloud.dc.scilifelab.se/public.php/webdav"
curl_upass = "zbC5fr2LbEZ9rSE:scRNAseq2025"
path_results = "data/trajectory"
if not os.path.exists(path_results):
os.makedirs(path_results, exist_ok=True)
path_file = "data/trajectory/trajectory_seurat_filtered.h5ad"
if not os.path.exists(path_file):
file_url = os.path.join(path_data, "trajectory/trajectory_seurat_filtered.h5ad")
subprocess.call(["curl", "-u", curl_upass, "-o", path_file, file_url ]) 3 Reading data
We already have pre-computed and subsetted the dataset (with 6688 cells and 3585 genes) following the analysis steps in this course. We then saved the objects, so you can use common tools to open and start to work with them (either in R or Python).
adata = sc.read_h5ad("data/trajectory/trajectory_seurat_filtered.h5ad")
adata.var| features | |
|---|---|
| 0610040J01Rik | 0610040J01Rik |
| 1190007I07Rik | 1190007I07Rik |
| 1500009L16Rik | 1500009L16Rik |
| 1700012B09Rik | 1700012B09Rik |
| 1700020L24Rik | 1700020L24Rik |
| ... | ... |
| Sqor | Sqor |
| Sting1 | Sting1 |
| Tent5a | Tent5a |
| Tlcd4 | Tlcd4 |
| Znrd2 | Znrd2 |
3585 rows × 1 columns
# check what you have in the X matrix, should be lognormalized counts.
print(adata.X[:10,:10])<Compressed Sparse Row sparse matrix of dtype 'float64'
with 25 stored elements and shape (10, 10)>
Coords Values
(0, 4) 0.11622072805743532
(0, 8) 0.4800893970571722
(1, 8) 0.2478910541698065
(1, 9) 0.17188973970230348
(2, 1) 0.09413397843954842
(2, 7) 0.18016412971724202
(3, 1) 0.08438841021254412
(3, 4) 0.08438841021254412
(3, 7) 0.08438841021254412
(3, 8) 0.3648216463668793
(4, 1) 0.14198147850903975
(4, 8) 0.14198147850903975
(5, 1) 0.17953169693896723
(5, 8) 0.17953169693896723
(5, 9) 0.17953169693896723
(6, 4) 0.2319546390006887
(6, 8) 0.42010741700351195
(7, 1) 0.1775659421407816
(7, 8) 0.39593115482156394
(7, 9) 0.09271901219711086
(8, 1) 0.12089079757716388
(8, 8) 0.22873058755480363
(9, 1) 0.08915380247493314
(9, 4) 0.08915380247493314
(9, 8) 0.382703987185901044 Explore the data
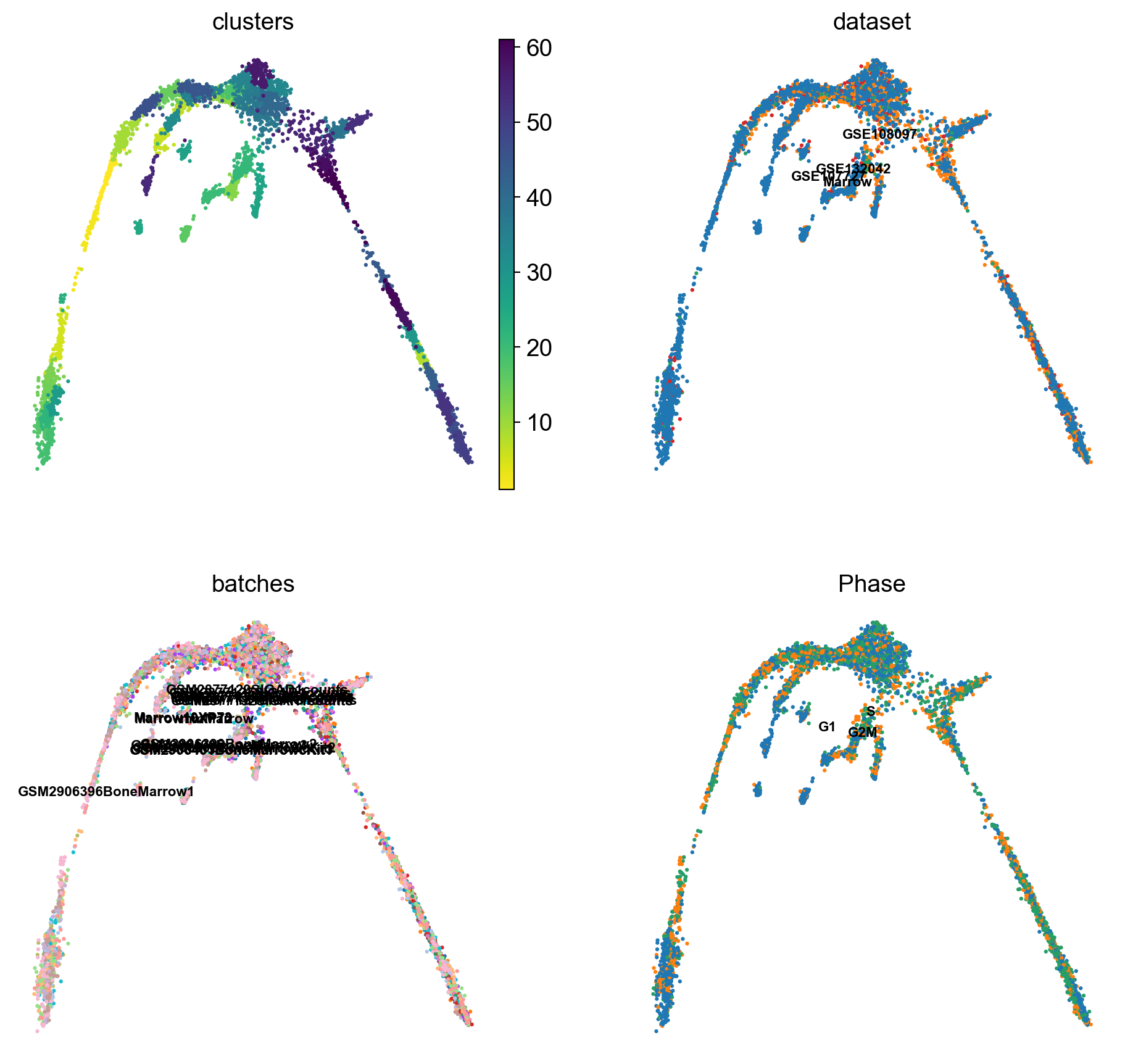
There is a umap and clusters provided with the object, first plot some information from the previous analysis onto the umap.
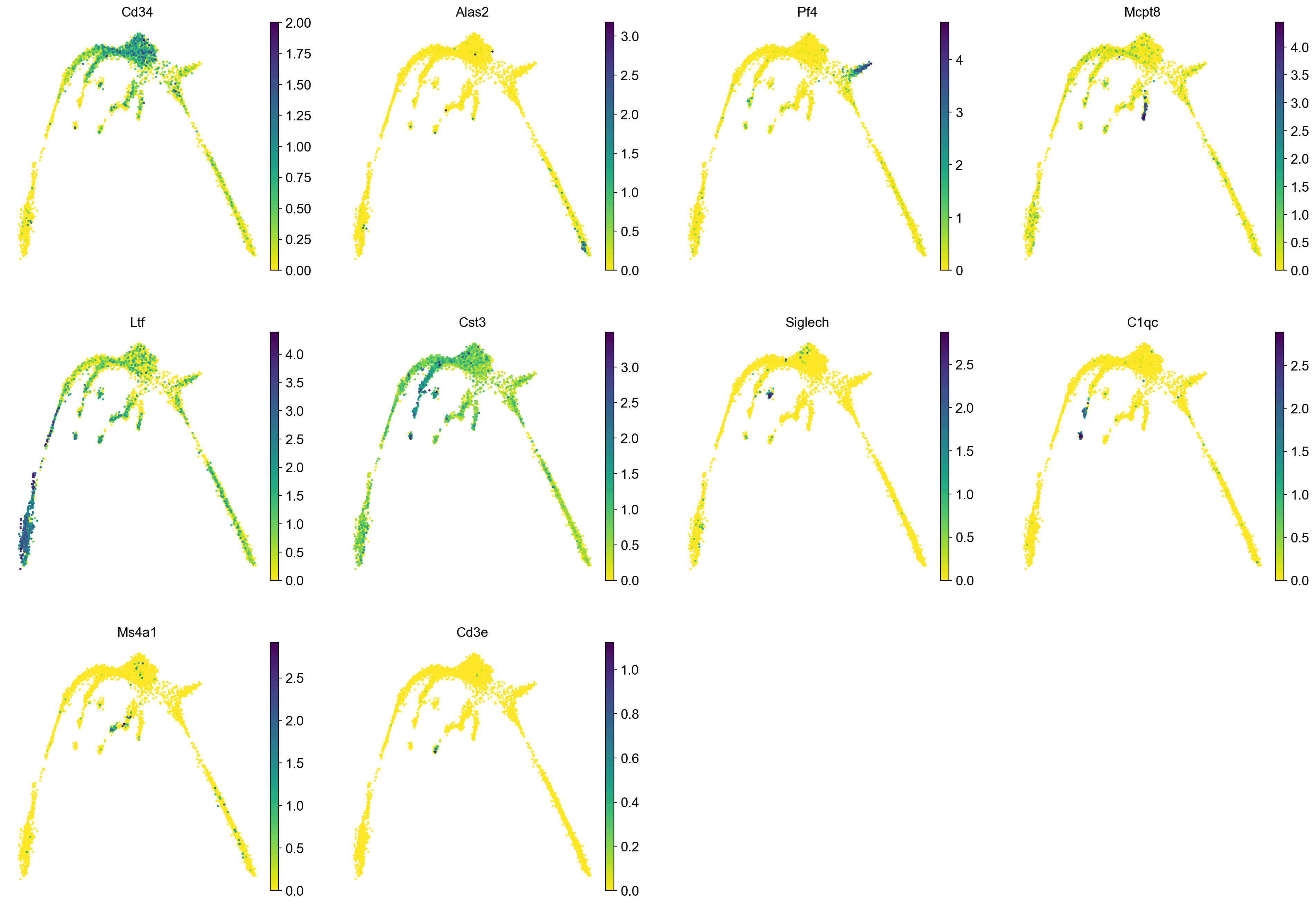
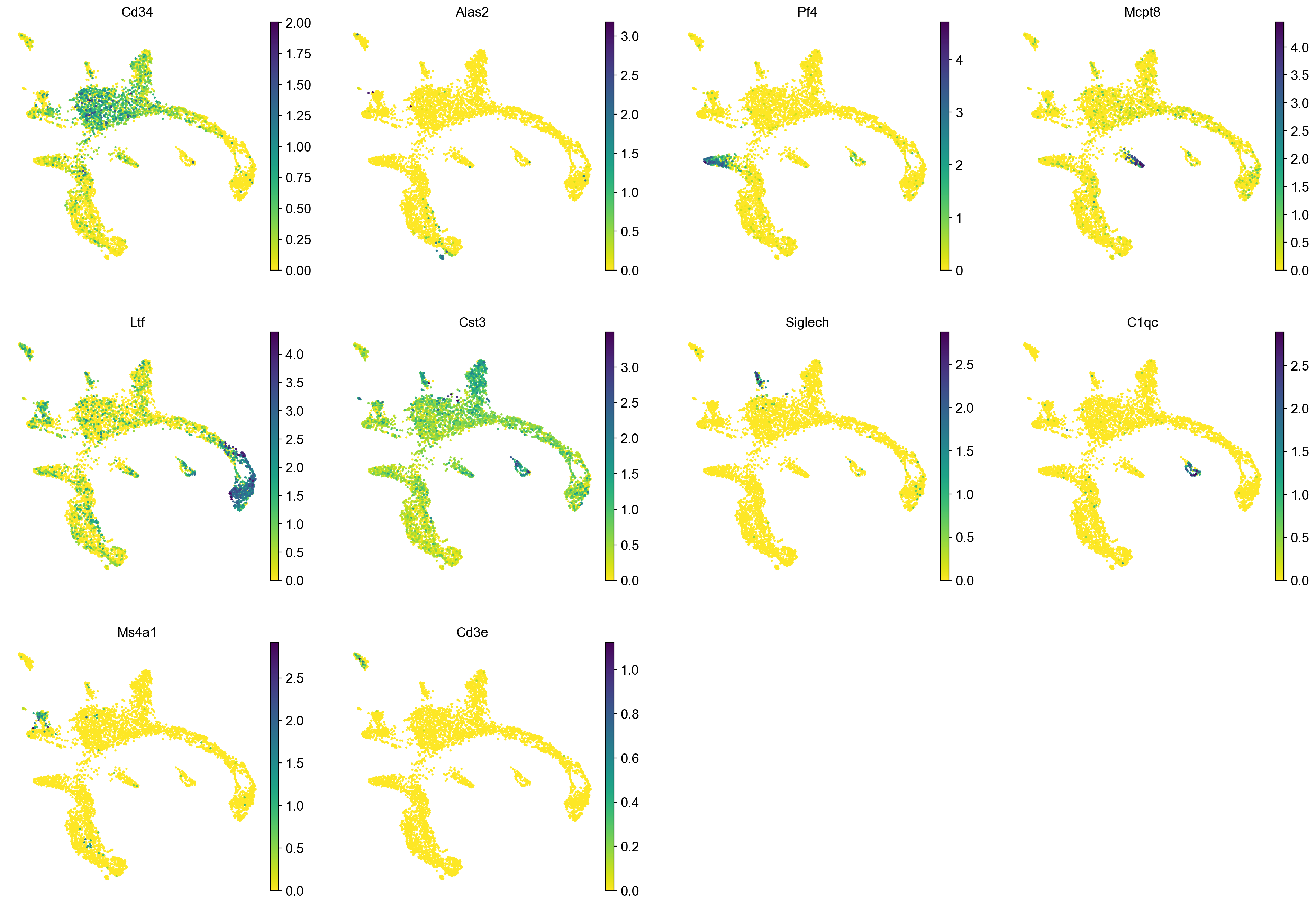
sc.pl.umap(adata, color = ['clusters','dataset','batches','Phase'],legend_loc = 'on data', legend_fontsize = 'xx-small', ncols = 2)It is crucial that you performing analysis of a dataset understands what is going on, what are the clusters you see in your data and most importantly How are the clusters related to each other?. Well, let’s explore the data a bit. With the help of this table, write down which cluster numbers in your dataset express these key markers.
| Marker | Cell Type |
|---|---|
| Cd34 | HSC progenitor |
| Ms4a1 | B cell lineage |
| Cd3e | T cell lineage |
| Ltf | Granulocyte lineage |
| Cst3 | Monocyte lineage |
| Mcpt8 | Mast Cell lineage |
| Alas2 | RBC lineage |
| Siglech | Dendritic cell lineage |
| C1qc | Macrophage cell lineage |
| Pf4 | Megakaryocyte cell lineage |
5 Rerun analysis in Scanpy
Redo clustering and umap using the basic Scanpy pipeline. Use the provided “X_harmony_Phase” dimensionality reduction as the staring point.
# first, store the old umap with a new name so it is not overwritten
adata.obsm['X_umap_old'] = adata.obsm['X_umap']
sc.pp.neighbors(adata, n_pcs = 30, n_neighbors = 20, use_rep="X_harmony_Phase")
sc.tl.umap(adata, min_dist=0.4, spread=3)computing neighbors
finished: added to `.uns['neighbors']`
`.obsp['distances']`, distances for each pair of neighbors
`.obsp['connectivities']`, weighted adjacency matrix (0:00:06)
computing UMAP
finished: added
'X_umap', UMAP coordinates (adata.obsm)
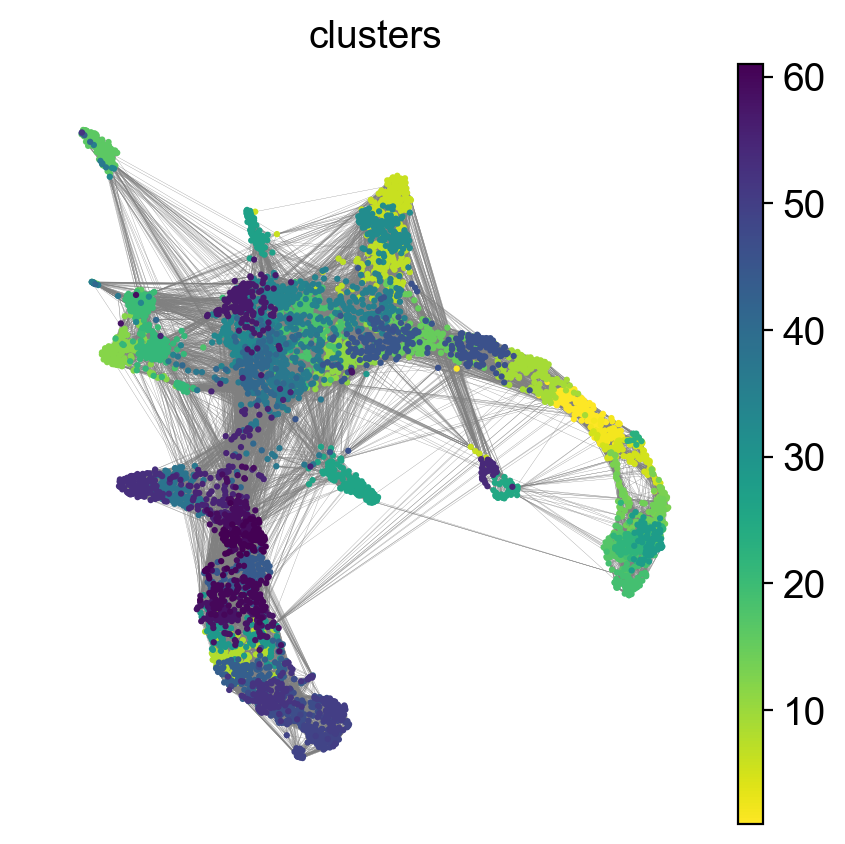
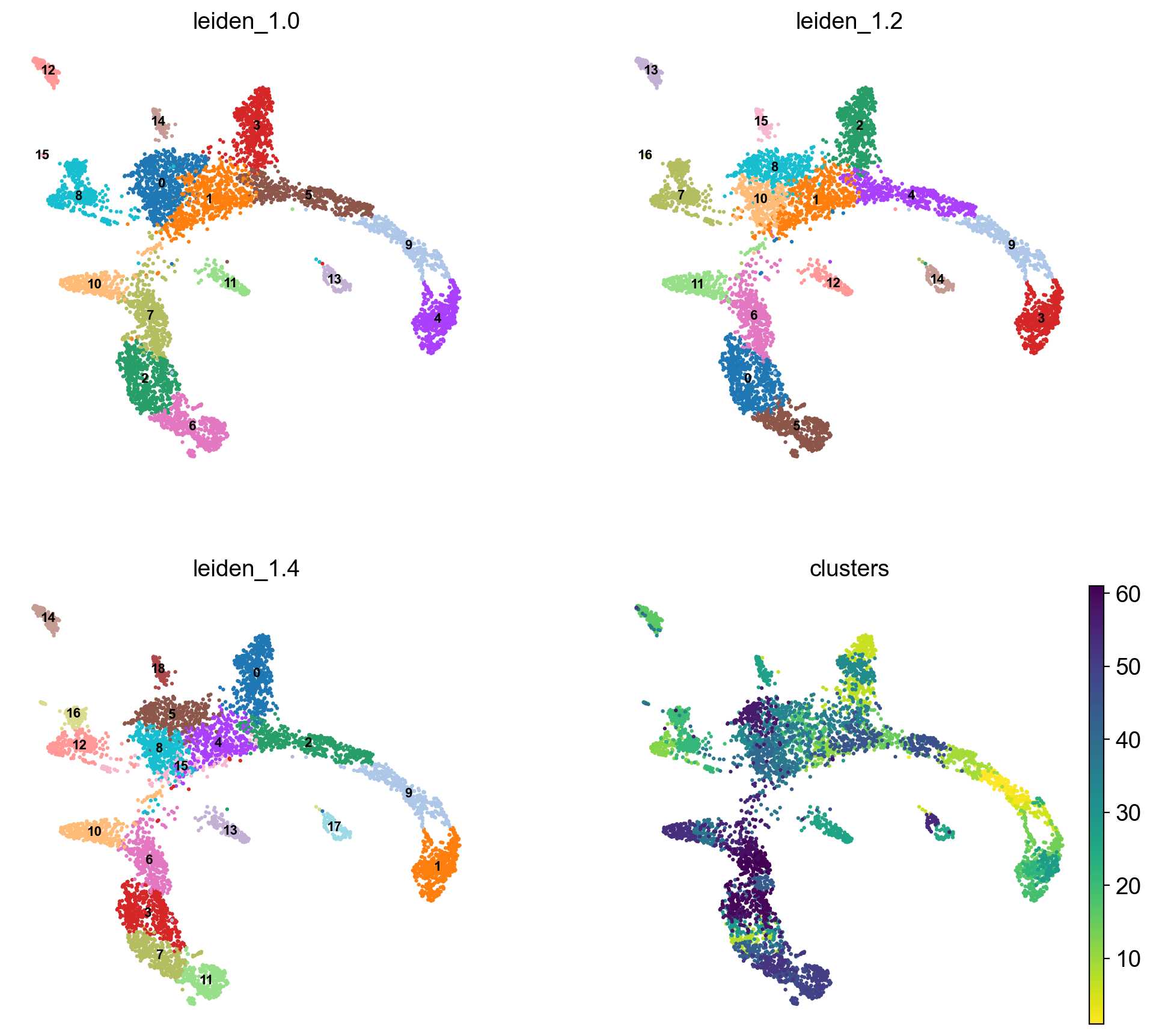
'umap', UMAP parameters (adata.uns) (0:00:10)sc.pl.umap(adata, color = ['clusters'],legend_loc = 'on data', legend_fontsize = 'xx-small', edges = True)
sc.pl.umap(adata, color = markers, use_raw = False, ncols = 4)
# Redo clustering as well
sc.tl.leiden(adata, key_added = "leiden_1.0", resolution = 1.0) # default resolution in 1.0
sc.tl.leiden(adata, key_added = "leiden_1.2", resolution = 1.2) # default resolution in 1.0
sc.tl.leiden(adata, key_added = "leiden_1.4", resolution = 1.4) # default resolution in 1.0
#sc.tl.louvain(adata, key_added = "leiden_1.0") # default resolution in 1.0
sc.pl.umap(adata, color = ['leiden_1.0', 'leiden_1.2', 'leiden_1.4','clusters'],legend_loc = 'on data', legend_fontsize = 'xx-small', ncols =2)running Leiden clustering
finished: found 16 clusters and added
'leiden_1.0', the cluster labels (adata.obs, categorical) (0:00:00)
running Leiden clustering
finished: found 17 clusters and added
'leiden_1.2', the cluster labels (adata.obs, categorical) (0:00:00)
running Leiden clustering
finished: found 19 clusters and added
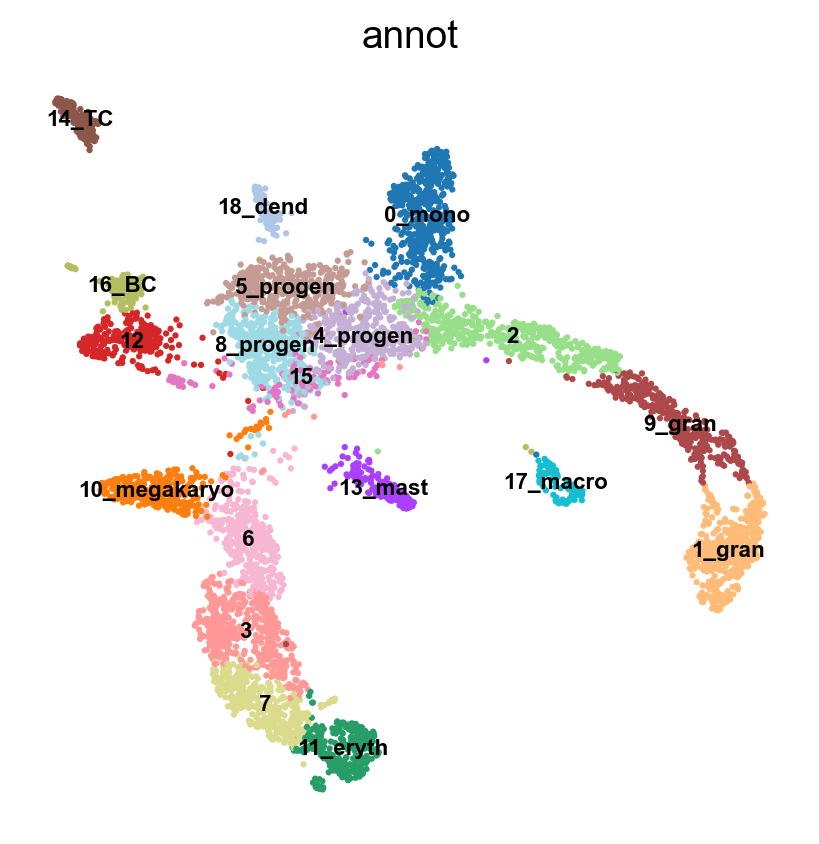
'leiden_1.4', the cluster labels (adata.obs, categorical) (0:00:00)#Rename clusters with really clear markers, the rest are left unlabelled.
annot = pd.DataFrame(adata.obs['leiden_1.4'].astype('string'))
annot[annot['leiden_1.4'] == '10'] = '10_megakaryo' #Pf4
annot[annot['leiden_1.4'] == '17'] = '17_macro' #C1qc
annot[annot['leiden_1.4'] == '11'] = '11_eryth' #Alas2
annot[annot['leiden_1.4'] == '18'] = '18_dend' #Siglech
annot[annot['leiden_1.4'] == '13'] = '13_mast' #Mcpt8
annot[annot['leiden_1.4'] == '0'] = '0_mono' #Cts3
annot[annot['leiden_1.4'] == '1'] = '1_gran' #Ltf
annot[annot['leiden_1.4'] == '9'] = '9_gran'
annot[annot['leiden_1.4'] == '14'] = '14_TC' #Cd3e
annot[annot['leiden_1.4'] == '16'] = '16_BC' #Ms4a1
annot[annot['leiden_1.4'] == '8'] = '8_progen' # Cd34
annot[annot['leiden_1.4'] == '4'] = '4_progen'
annot[annot['leiden_1.4'] == '5'] = '5_progen'
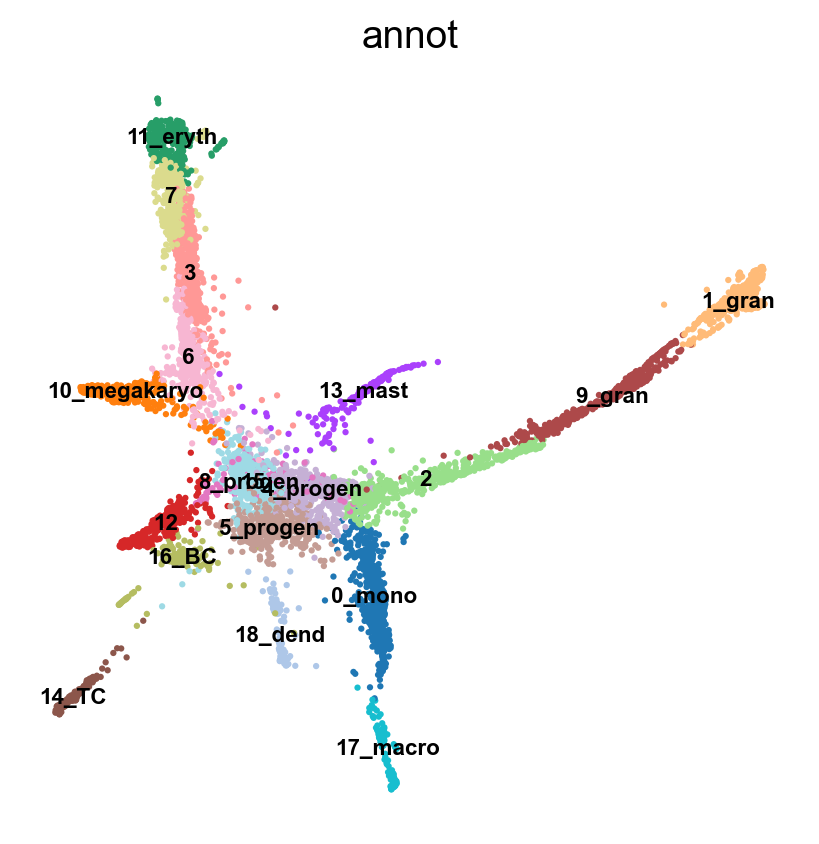
adata.obs['annot']=annot['leiden_1.4'].astype('category')
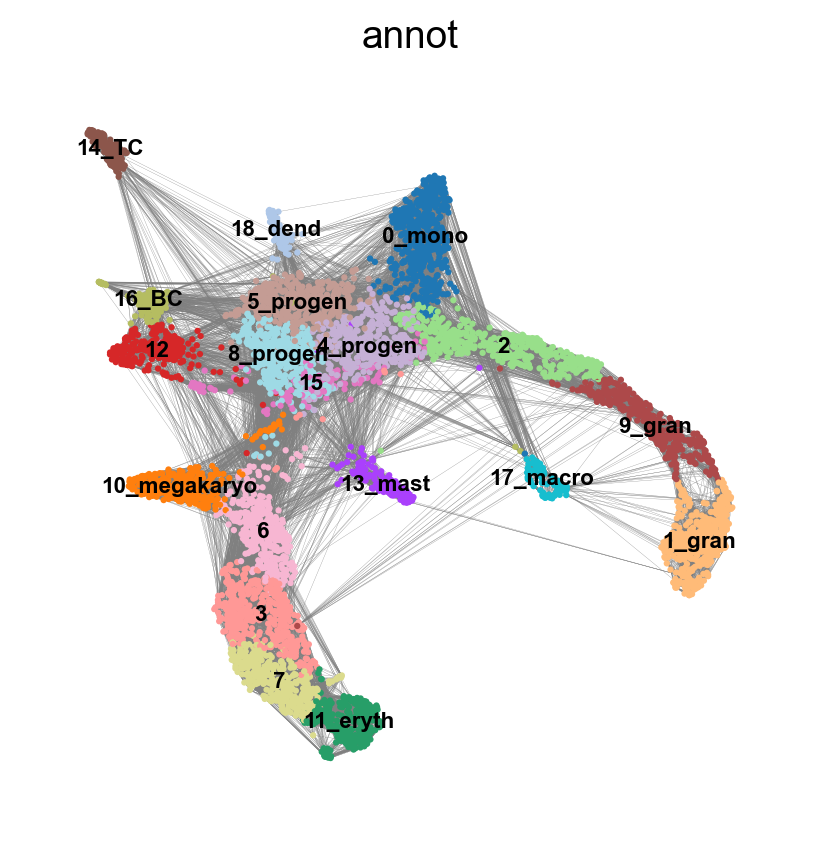
sc.pl.umap(adata, color = 'annot',legend_loc = 'on data', legend_fontsize = 'xx-small', ncols =2)
annot.value_counts()
#type(annot)
# astype('category')leiden_1.4
0_mono 509
1_gran 487
2 479
3 463
4_progen 387
5_progen 384
7 368
6 368
8_progen 367
9_gran 366
10_megakaryo 301
11_eryth 294
12 276
13_mast 159
14_TC 151
15 128
16_BC 124
17_macro 116
18_dend 101
dtype: int646 Run PAGA
Use the clusters from leiden clustering with leiden_1.4 and run PAGA. First we create the graph and initialize the positions using the umap.
# use the umap to initialize the graph layout.
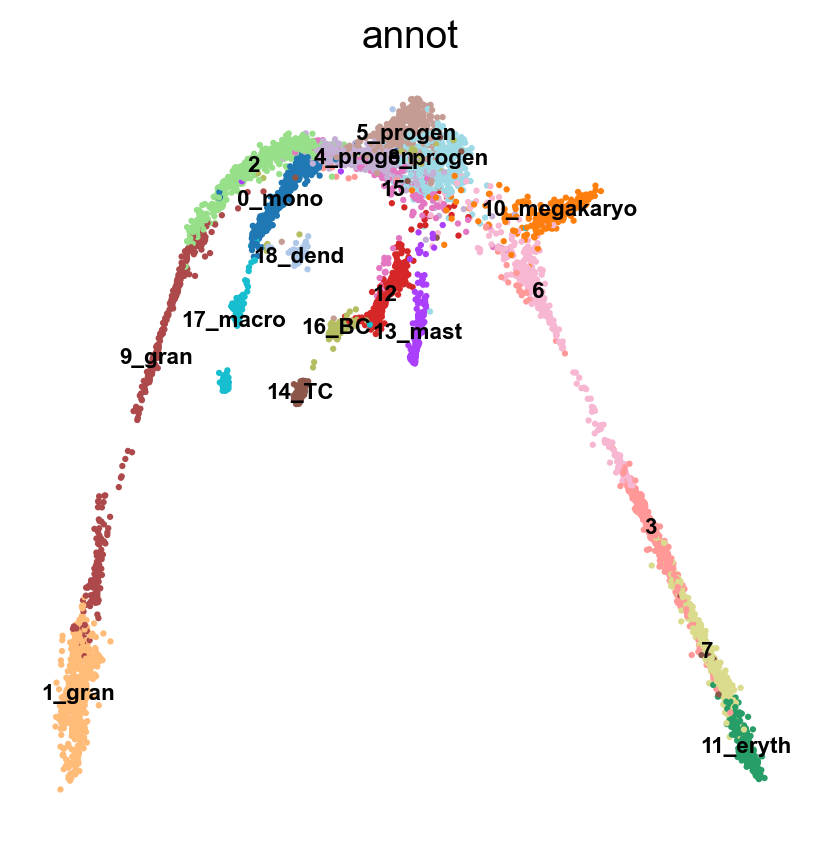
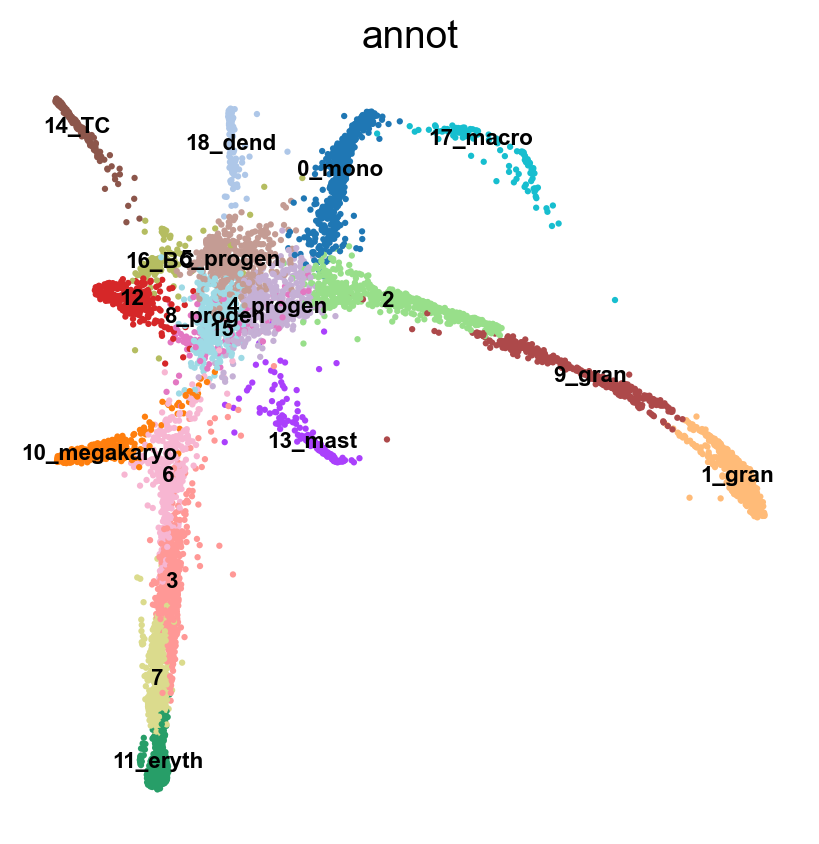
sc.tl.draw_graph(adata, init_pos='X_umap')
sc.pl.draw_graph(adata, color='annot', legend_loc='on data', legend_fontsize = 'xx-small')
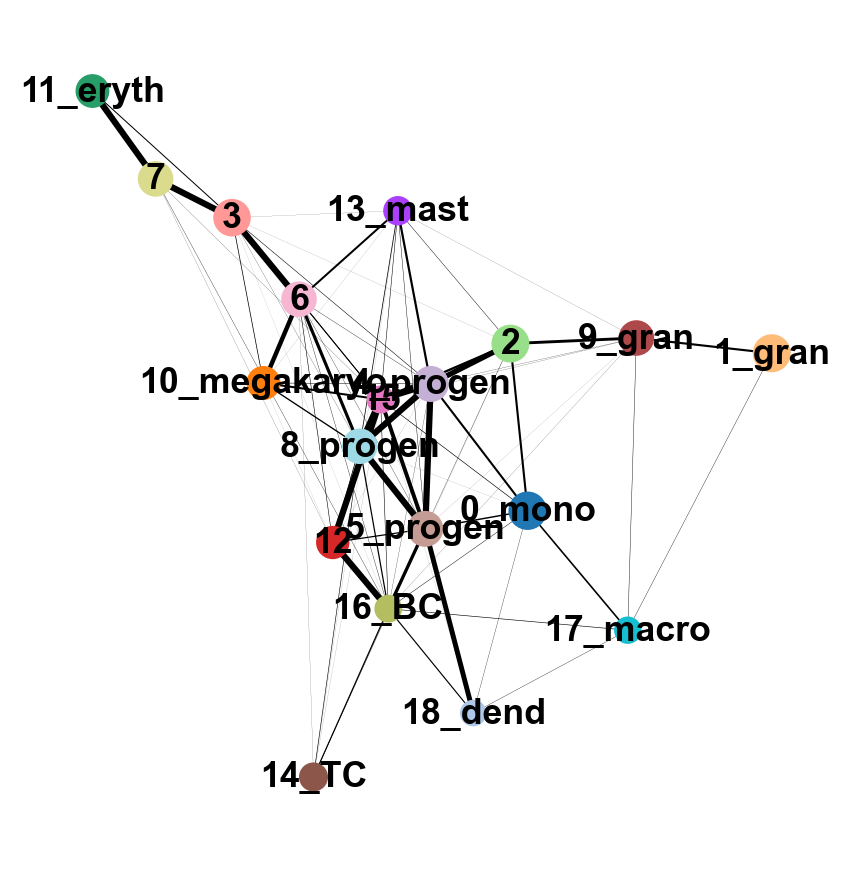
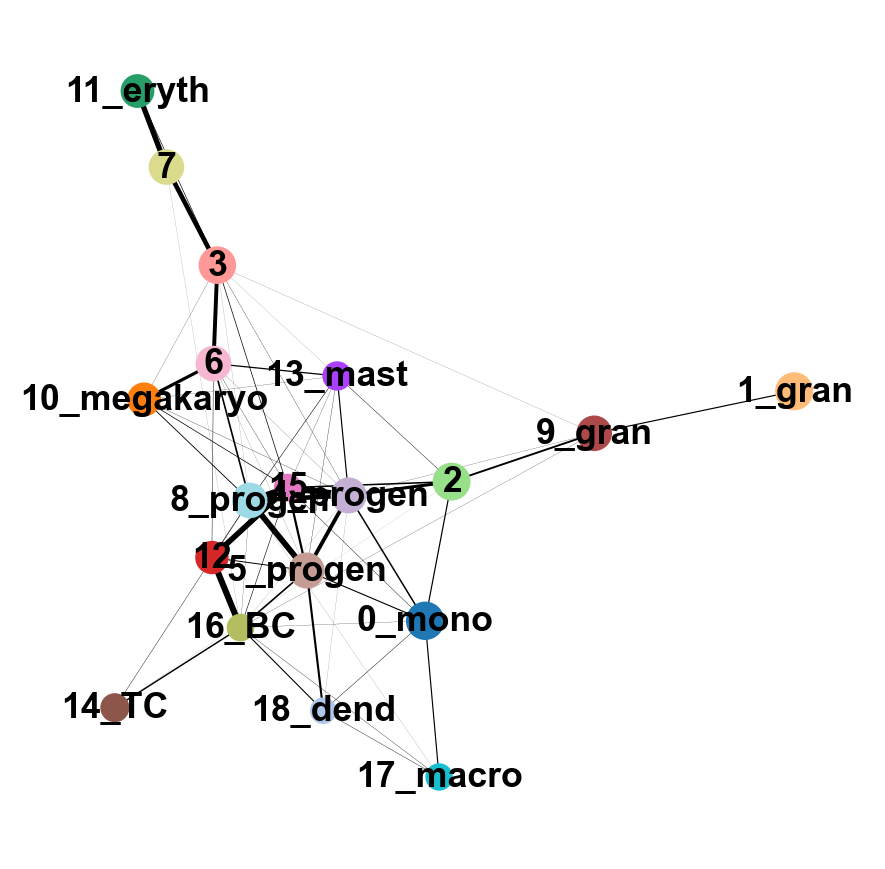
sc.tl.paga(adata, groups='annot')
sc.pl.paga(adata, color='annot', edge_width_scale = 0.3)drawing single-cell graph using layout 'fa'
finished: added
'X_draw_graph_fa', graph_drawing coordinates (adata.obsm) (0:00:29)running PAGA
finished: added
'paga/connectivities', connectivities adjacency (adata.uns)
'paga/connectivities_tree', connectivities subtree (adata.uns) (0:00:00)
--> added 'pos', the PAGA positions (adata.uns['paga'])As you can see, we have edges between many clusters that we know are are unrelated, so we may need to clean up the data a bit more.
7 Filtering graph edges
First, lets explore the graph a bit. So we plot the umap with the graph connections on top.
We have many edges in the graph between unrelated clusters, so lets try with fewer neighbors.
sc.pp.neighbors(adata, n_neighbors=5, use_rep = 'X_harmony_Phase', n_pcs = 30)
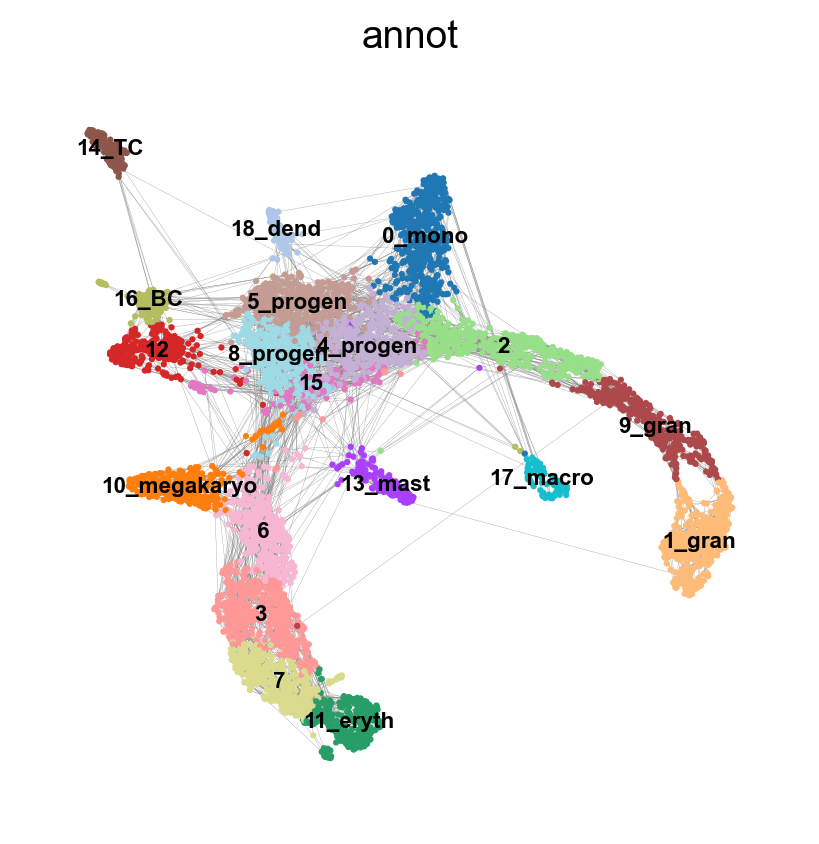
sc.pl.umap(adata, edges=True, color = 'annot', legend_loc= 'on data', legend_fontsize= 'xx-small')computing neighbors
finished: added to `.uns['neighbors']`
`.obsp['distances']`, distances for each pair of neighbors
`.obsp['connectivities']`, weighted adjacency matrix (0:00:00)7.1 Rerun PAGA again on the data
sc.tl.draw_graph(adata, init_pos='X_umap')
sc.pl.draw_graph(adata, color='annot', legend_loc='on data', legend_fontsize = 'xx-small')drawing single-cell graph using layout 'fa'
finished: added
'X_draw_graph_fa', graph_drawing coordinates (adata.obsm) (0:00:27)sc.tl.paga(adata, groups='annot')
sc.pl.paga(adata, color='annot', edge_width_scale = 0.3)running PAGA
finished: added
'paga/connectivities', connectivities adjacency (adata.uns)
'paga/connectivities_tree', connectivities subtree (adata.uns) (0:00:00)
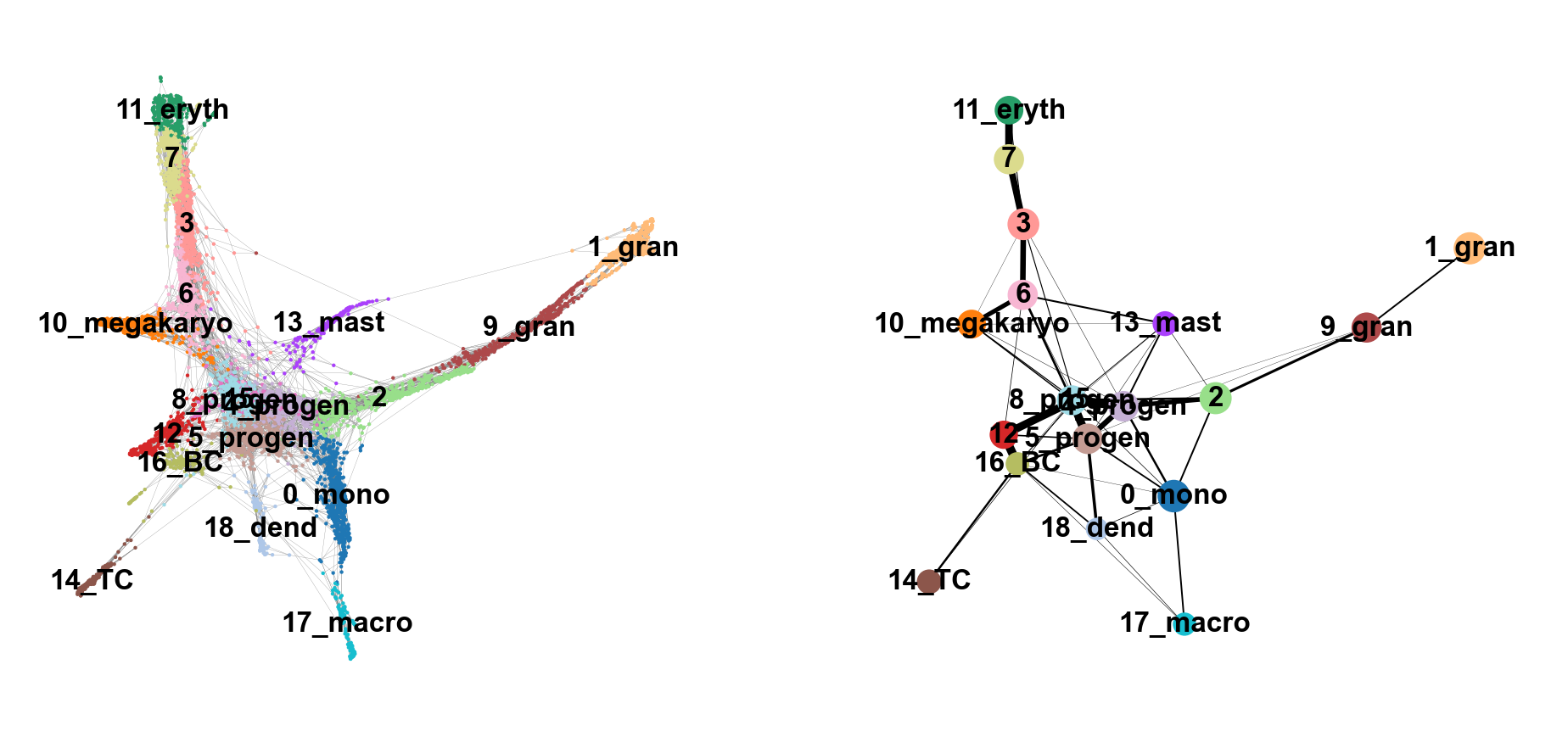
--> added 'pos', the PAGA positions (adata.uns['paga'])8 Embedding using PAGA-initialization
We can now redraw the graph using another starting position from the paga layout. The following is just as well possible for a UMAP.
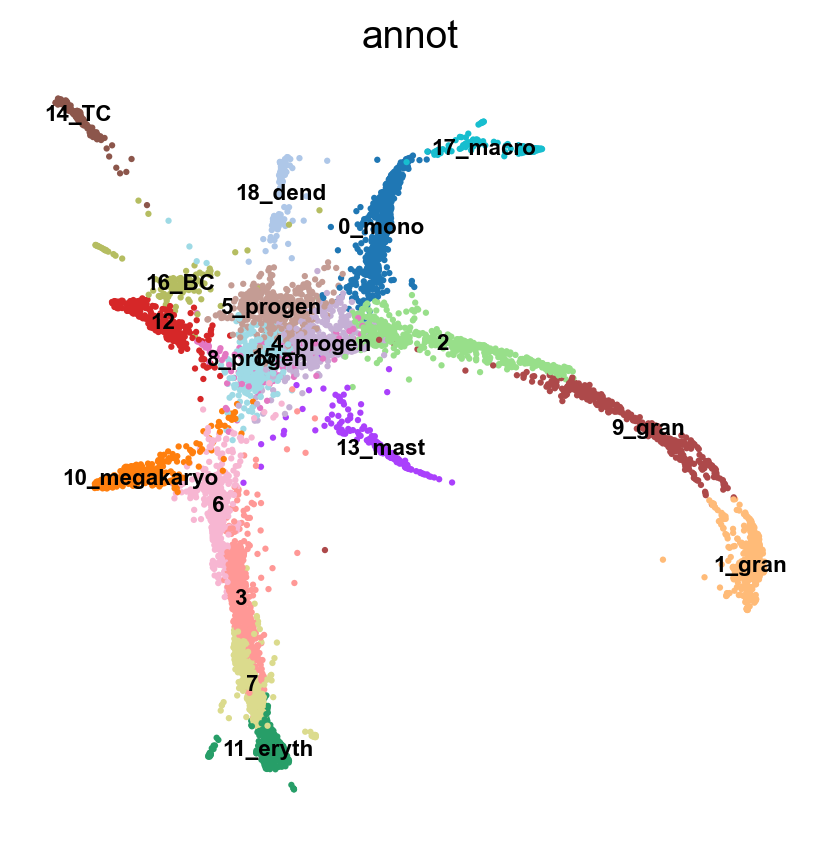
sc.tl.draw_graph(adata, init_pos='paga')drawing single-cell graph using layout 'fa'
finished: added
'X_draw_graph_fa', graph_drawing coordinates (adata.obsm) (0:00:28)Now we can see all marker genes also at single-cell resolution in a meaningful layout.
Compare the 2 graphs
sc.pl.paga_compare(
adata, threshold=0.03, title='', right_margin=0.2, size=10, edge_width_scale=0.5,
legend_fontsize=12, fontsize=12, frameon=False, edges=True)--> added 'pos', the PAGA positions (adata.uns['paga'])Does this graph fit the biological expectations given what you know of hematopoesis. Please have a look at the figure in Section 2 and compare to the paths you now have.
9 Gene changes
We can reconstruct gene changes along PAGA paths for a given set of genes
Choose a root cell for diffusion pseudotime. We have 3 progenitor clusters, but cluster 5 seems the most clear.
adata.uns['iroot'] = np.flatnonzero(adata.obs['annot'] == '5_progen')[0]
sc.tl.dpt(adata)WARNING: Trying to run `tl.dpt` without prior call of `tl.diffmap`. Falling back to `tl.diffmap` with default parameters.
computing Diffusion Maps using n_comps=15(=n_dcs)
computing transitions
finished (0:00:00)
eigenvalues of transition matrix
[1. 0.9989591 0.997628 0.9970365 0.9956704 0.99334306
0.9918951 0.9915921 0.99013233 0.98801893 0.9870309 0.9861044
0.9851118 0.9845008 0.9839531 ]
finished: added
'X_diffmap', diffmap coordinates (adata.obsm)
'diffmap_evals', eigenvalues of transition matrix (adata.uns) (0:00:01)
computing Diffusion Pseudotime using n_dcs=10
finished: added
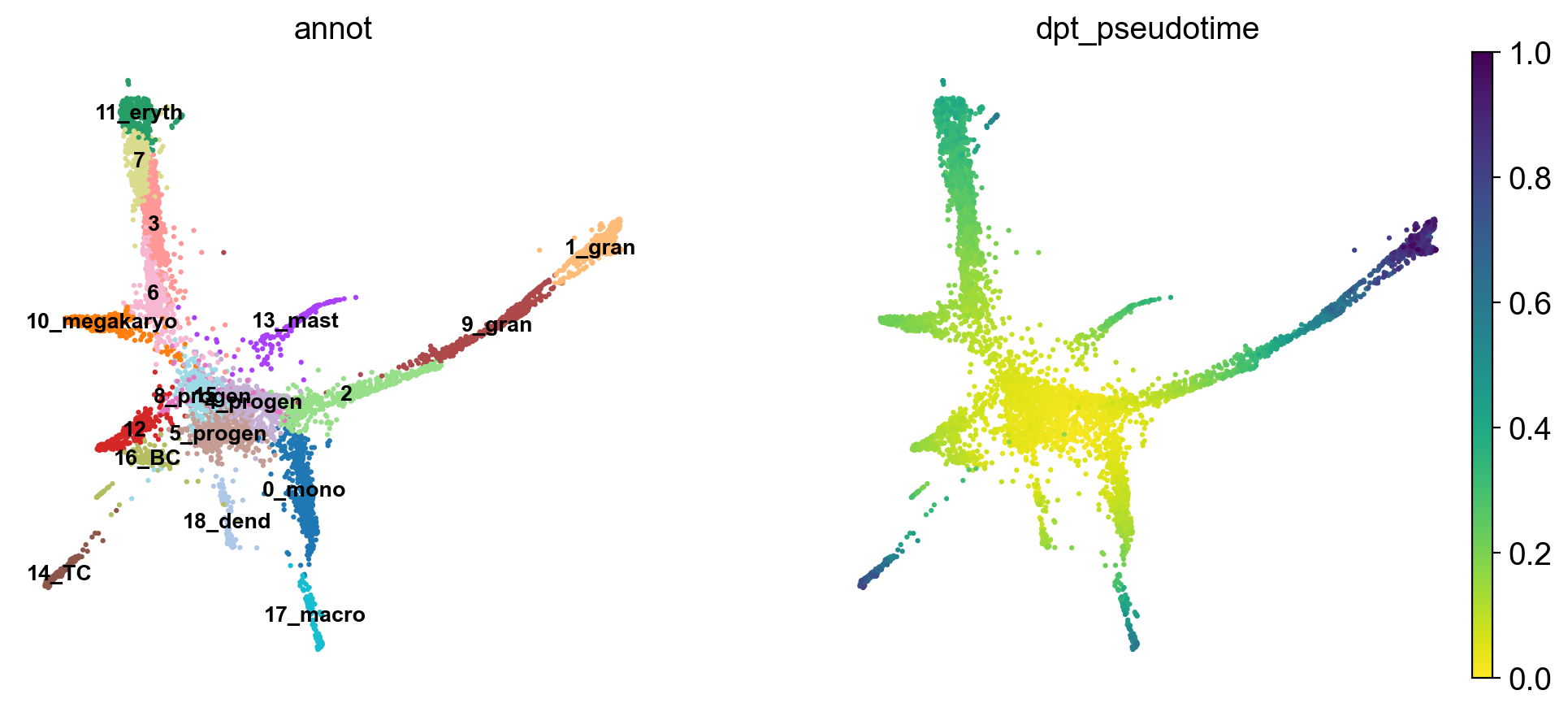
'dpt_pseudotime', the pseudotime (adata.obs) (0:00:00)Use the full raw data for visualization.
sc.pl.draw_graph(adata, color=['annot', 'dpt_pseudotime'], legend_loc='on data', legend_fontsize= 'x-small')The pseudotime represents the distance of every cell to the starting cluster. Have a look at the pseudotime plot, how well do you think it represents actual developmental time? What does it represent?
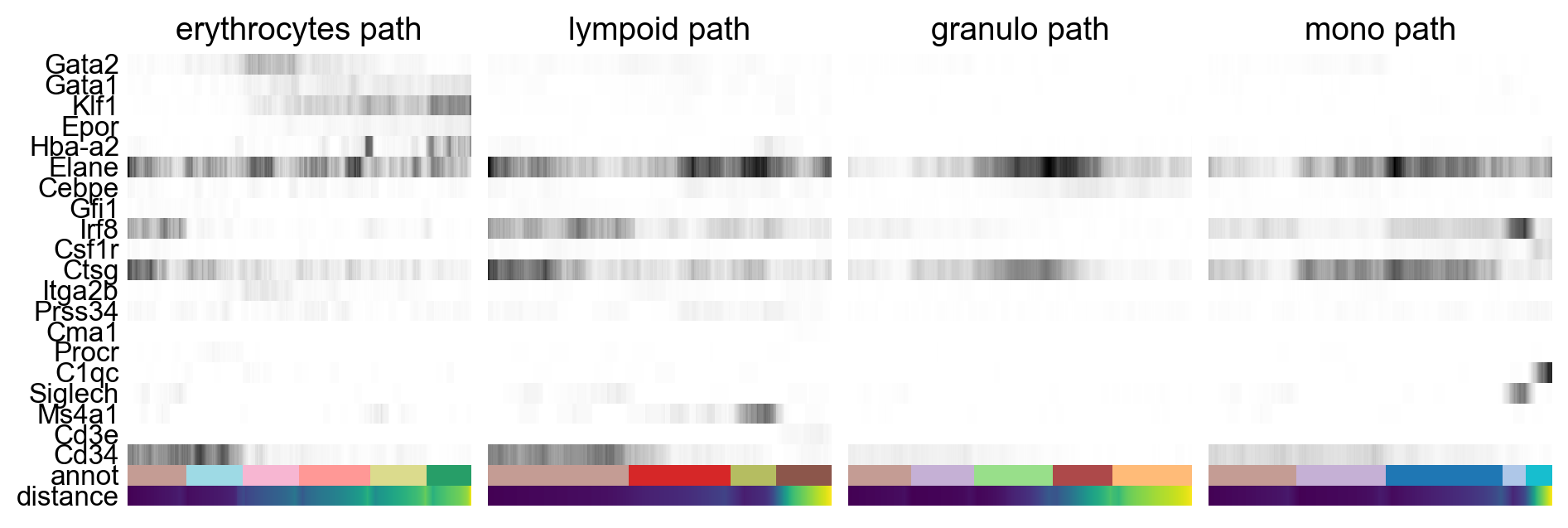
By looking at the different know lineages and the layout of the graph we define manually some paths to the graph that corresponds to specific lineages.
# Define paths
paths = [('erythrocytes', ['5_progen', '8_progen', '6', '3', '7', '11_eryth']),
('lympoid', ['5_progen', '12', '16_BC', '14_TC']),
('granulo', ['5_progen', '4_progen', '2', '9_gran', '1_gran']),
('mono', ['5_progen', '4_progen', '0_mono', '18_dend', '17_macro'])
]
adata.obs['distance'] = adata.obs['dpt_pseudotime']Then we select some genes that can vary in the lineages and plot onto the paths.
gene_names = ['Gata2', 'Gata1', 'Klf1', 'Epor', 'Hba-a2', # erythroid
'Elane', 'Cebpe', 'Gfi1', # neutrophil
'Irf8', 'Csf1r', 'Ctsg', # monocyte
'Itga2b','Prss34','Cma1','Procr', # Megakaryo,Basophil,Mast,HPC
'C1qc','Siglech','Ms4a1','Cd3e','Cd34']_, axs = pl.subplots(ncols=4, figsize=(10, 4), gridspec_kw={
'wspace': 0.05, 'left': 0.12})
pl.subplots_adjust(left=0.05, right=0.98, top=0.82, bottom=0.2)
for ipath, (descr, path) in enumerate(paths):
sc.pl.paga_path(
adata=adata,
nodes=path,
keys=gene_names,
show_node_names=False,
ax=axs[ipath],
ytick_fontsize=12,
left_margin=0.15,
n_avg=50,
annotations=['distance'],
show_yticks=True if ipath == 0 else False,
show_colorbar=False,
color_map='Greys',
groups_key='annot',
color_maps_annotations={'distance': 'viridis'},
title='{} path'.format(descr),
return_data=True,
use_raw=False,
show=False)
pl.show()As you can see, we can manipulate the trajectory quite a bit by selecting different number of neighbors, components etc. to fit with our assumptions on the development of these celltypes.
Please explore further how you can tweak the trajectory. For instance, can you create a PAGA trajectory using the orignial umap from Seurat instead? Hint, you first need to compute the neighbors on the umap.
10 Session info
Click here
sc.logging.print_versions()-----
anndata 0.10.8
scanpy 1.10.3
-----
CoreFoundation NA
Foundation NA
PIL 10.4.0
PyObjCTools NA
anyio NA
appnope 0.1.4
arrow 1.3.0
asciitree NA
asttokens NA
attr 24.2.0
attrs 24.2.0
babel 2.14.0
brotli 1.1.0
certifi 2024.08.30
cffi 1.17.1
charset_normalizer 3.3.2
cloudpickle 3.0.0
colorama 0.4.6
comm 0.2.2
cycler 0.12.1
cython_runtime NA
cytoolz 0.12.3
dask 2024.9.0
dateutil 2.9.0
debugpy 1.8.5
decorator 5.1.1
defusedxml 0.7.1
exceptiongroup 1.2.2
executing 2.1.0
fa2_modified NA
fastjsonschema NA
fqdn NA
google NA
h5py 3.11.0
idna 3.10
igraph 0.11.6
ipykernel 6.29.5
isoduration NA
jedi 0.19.1
jinja2 3.1.4
joblib 1.4.2
json5 0.9.25
jsonpointer 3.0.0
jsonschema 4.23.0
jsonschema_specifications NA
jupyter_events 0.10.0
jupyter_server 2.14.2
jupyterlab_server 2.27.3
kiwisolver 1.4.7
legacy_api_wrap NA
leidenalg 0.10.2
llvmlite 0.43.0
markupsafe 2.1.5
matplotlib 3.10.0
matplotlib_inline 0.1.7
mpl_toolkits NA
msgpack 1.1.0
natsort 8.4.0
nbformat 5.10.4
networkx 3.3
numba 0.60.0
numcodecs 0.13.0
numpy 1.26.4
objc 10.3.1
overrides NA
packaging 24.1
pandas 1.5.3
parso 0.8.4
pickleshare 0.7.5
platformdirs 4.3.6
prometheus_client NA
prompt_toolkit 3.0.47
psutil 6.0.0
pure_eval 0.2.3
pycparser 2.22
pydev_ipython NA
pydevconsole NA
pydevd 2.9.5
pydevd_file_utils NA
pydevd_plugins NA
pydevd_tracing NA
pygments 2.18.0
pynndescent 0.5.13
pyparsing 3.1.4
pythonjsonlogger NA
pytz 2024.2
referencing NA
requests 2.32.3
rfc3339_validator 0.1.4
rfc3986_validator 0.1.1
rpds NA
scipy 1.14.1
send2trash NA
session_info 1.0.0
six 1.16.0
sklearn 1.1.3
sniffio 1.3.1
socks 1.7.1
sparse 0.15.4
sphinxcontrib NA
stack_data 0.6.2
texttable 1.7.0
threadpoolctl 3.5.0
tlz 0.12.3
toolz 0.12.1
torch 2.4.0.post101
torchgen NA
tornado 6.4.1
tqdm 4.66.5
traitlets 5.14.3
typing_extensions NA
umap 0.5.6
uri_template NA
urllib3 2.3.0
wcwidth 0.2.13
webcolors 24.8.0
websocket 1.8.0
yaml 6.0.2
zarr 2.18.3
zipp NA
zmq 26.2.0
zoneinfo NA
zstandard 0.23.0
-----
IPython 8.27.0
jupyter_client 8.6.3
jupyter_core 5.7.2
jupyterlab 4.3.5
notebook 7.3.2
-----
Python 3.10.10 | packaged by conda-forge | (main, Mar 24 2023, 20:17:34) [Clang 14.0.6 ]
macOS-15.3.2-x86_64-i386-64bit
-----
Session information updated at 2025-04-03 12:01