import numpy as np
import pandas as pd
import scanpy as sc
import matplotlib.pyplot as plt
import warnings
import os
import subprocess
warnings.simplefilter(action="ignore", category=Warning)
# verbosity: errors (0), warnings (1), info (2), hints (3)
sc.settings.verbosity = 2
sc.settings.set_figure_params(dpi=80)Code chunks run Python commands unless it starts with %%bash, in which case, those chunks run shell commands.
Celltype prediction can either be performed on indiviudal cells where each cell gets a predicted celltype label, or on the level of clusters. All methods are based on similarity to other datasets, single cell or sorted bulk RNAseq, or uses known marker genes for each cell type.
Ideally celltype predictions should be run on each sample separately and not using the integrated data. In this case we will select one sample from the Covid data, ctrl_13 and predict celltype by cell on that sample.
Some methods will predict a celltype to each cell based on what it is most similar to, even if that celltype is not included in the reference. Other methods include an uncertainty so that cells with low similarity scores will be unclassified.
There are multiple different methods to predict celltypes, here we will just cover a few of those.
Here we will use a reference PBMC dataset that we get from scanpy datasets and classify celltypes based on two methods:
- Using scanorama for integration just as in the integration lab, and then do label transfer based on closest neighbors.
- Using ingest to project the data onto the reference data and transfer labels.
- Using Celltypist to predicted with a pretrained pbmc model or with an own model based on the same reference data as the other methods.
First, lets load required libraries
Let’s read in the saved Covid-19 data object from the clustering step.
# download pre-computed data if missing or long compute
fetch_data = True
# url for source and intermediate data
path_data = "https://nextcloud.dc.scilifelab.se/public.php/webdav"
curl_upass = "zbC5fr2LbEZ9rSE:scRNAseq2025"
path_results = "data/covid/results"
if not os.path.exists(path_results):
os.makedirs(path_results, exist_ok=True)
path_file = "data/covid/results/scanpy_covid_qc_dr_int_cl.h5ad"
if fetch_data and not os.path.exists(path_file):
file_url = os.path.join(path_data, "covid/results_scanpy/scanpy_covid_qc_dr_int_cl.h5ad")
subprocess.call(["curl", "-u", curl_upass, "-o", path_file, file_url ])
adata = sc.read_h5ad(path_file)
adataAnnData object with n_obs × n_vars = 7332 × 19468
obs: 'type', 'sample', 'batch', 'n_genes_by_counts', 'total_counts', 'total_counts_mt', 'pct_counts_mt', 'total_counts_ribo', 'pct_counts_ribo', 'total_counts_hb', 'pct_counts_hb', 'percent_mt2', 'n_counts', 'n_genes', 'percent_chrY', 'XIST-counts', 'S_score', 'G2M_score', 'phase', 'doublet_scores', 'predicted_doublets', 'doublet_info', 'leiden', 'leiden_0.4', 'leiden_0.6', 'leiden_1.0', 'leiden_1.4', 'kmeans5', 'kmeans10', 'kmeans15', 'hclust_5', 'hclust_10', 'hclust_15'
var: 'gene_ids', 'feature_types', 'genome', 'mt', 'ribo', 'hb', 'n_cells_by_counts', 'mean_counts', 'pct_dropout_by_counts', 'total_counts', 'n_cells', 'highly_variable', 'means', 'dispersions', 'dispersions_norm', 'highly_variable_nbatches', 'highly_variable_intersection'
uns: 'dendrogram_leiden_0.6', 'doublet_info_colors', 'hclust_10_colors', 'hclust_15_colors', 'hclust_5_colors', 'hvg', 'kmeans10_colors', 'kmeans15_colors', 'kmeans5_colors', 'leiden', 'leiden_0.4', 'leiden_0.4_colors', 'leiden_0.6', 'leiden_0.6_colors', 'leiden_1.0', 'leiden_1.0_colors', 'leiden_1.4', 'leiden_1.4_colors', 'log1p', 'neighbors', 'pca', 'phase_colors', 'sample_colors', 'tsne', 'umap'
obsm: 'Scanorama', 'X_pca', 'X_pca_harmony', 'X_tsne', 'X_tsne_bbknn', 'X_tsne_harmony', 'X_tsne_scanorama', 'X_tsne_uncorr', 'X_umap', 'X_umap_bbknn', 'X_umap_harmony', 'X_umap_scanorama', 'X_umap_uncorr'
obsp: 'connectivities', 'distances'adata.uns['log1p']['base']=None
print(adata.shape)(7332, 19468)Subset one patient.
adata = adata[adata.obs["sample"] == "ctrl_13",:]
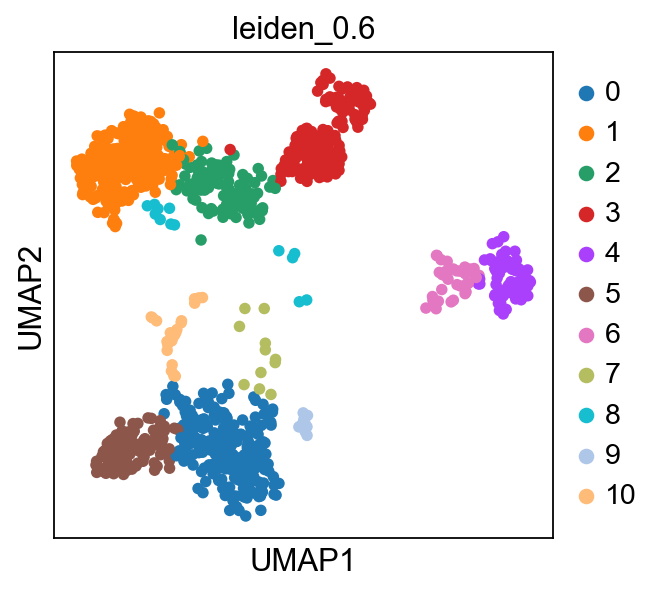
print(adata.shape)(1154, 19468)adata.obs["leiden_0.6"].value_counts()0 269
5 210
1 134
6 132
2 123
4 112
3 100
7 24
10 20
9 16
8 14
Name: leiden_0.6, dtype: int64Some clusters have very few cells from this individual, so any cluster comparisons may be biased by this.
1 Reference data
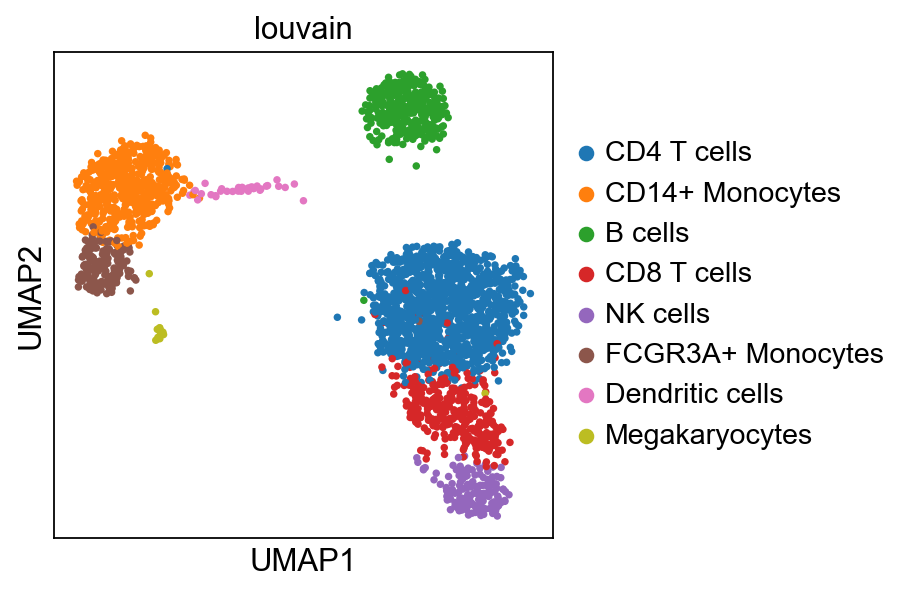
Load the reference data from scanpy.datasets. It is the annotated and processed pbmc3k dataset from 10x.
adata_ref = sc.datasets.pbmc3k_processed()
adata_ref.obs['sample']='pbmc3k'
print(adata_ref.shape)
adata_ref.obs(2638, 1838)| n_genes | percent_mito | n_counts | louvain | sample | |
|---|---|---|---|---|---|
| index | |||||
| AAACATACAACCAC-1 | 781 | 0.030178 | 2419.0 | CD4 T cells | pbmc3k |
| AAACATTGAGCTAC-1 | 1352 | 0.037936 | 4903.0 | B cells | pbmc3k |
| AAACATTGATCAGC-1 | 1131 | 0.008897 | 3147.0 | CD4 T cells | pbmc3k |
| AAACCGTGCTTCCG-1 | 960 | 0.017431 | 2639.0 | CD14+ Monocytes | pbmc3k |
| AAACCGTGTATGCG-1 | 522 | 0.012245 | 980.0 | NK cells | pbmc3k |
| ... | ... | ... | ... | ... | ... |
| TTTCGAACTCTCAT-1 | 1155 | 0.021104 | 3459.0 | CD14+ Monocytes | pbmc3k |
| TTTCTACTGAGGCA-1 | 1227 | 0.009294 | 3443.0 | B cells | pbmc3k |
| TTTCTACTTCCTCG-1 | 622 | 0.021971 | 1684.0 | B cells | pbmc3k |
| TTTGCATGAGAGGC-1 | 454 | 0.020548 | 1022.0 | B cells | pbmc3k |
| TTTGCATGCCTCAC-1 | 724 | 0.008065 | 1984.0 | CD4 T cells | pbmc3k |
2638 rows × 5 columns
As you can see, the celltype annotation is in the metadata column louvain, so that is the column we will have to use for classification.
Make sure we have the same genes in both datset by taking the intersection
# before filtering genes, store the full matrix in raw.
adata.raw = adata
# also store the umap in a new slot as it will get overwritten
adata.obsm["X_umap_uncorr"] = adata.obsm["X_umap"]
print(adata_ref.shape[1])
print(adata.shape[1])
var_names = adata_ref.var_names.intersection(adata.var_names)
print(len(var_names))
adata_ref = adata_ref[:, var_names]
adata = adata[:, var_names]1838
19468
1676First we need to rerun pca and umap with the same gene set for both datasets.
sc.pp.pca(adata_ref)
sc.pp.neighbors(adata_ref)
sc.tl.umap(adata_ref)
sc.pl.umap(adata_ref, color='louvain')computing PCA
with n_comps=50
finished (0:00:00)
computing neighbors
using 'X_pca' with n_pcs = 50
finished (0:00:10)
computing UMAP
finished (0:00:04)2 Integrate with scanorama
import scanorama
#subset the individual dataset to the same variable genes as in MNN-correct.
alldata = dict()
alldata['ctrl']=adata
alldata['ref']=adata_ref
#convert to list of AnnData objects
adatas = list(alldata.values())
# run scanorama.integrate
scanorama.integrate_scanpy(adatas, dimred = 50)Found 1676 genes among all datasets
[[0. 0.42894281]
[0. 0. ]]
Processing datasets (0, 1)# add in sample info
adata_ref.obs['sample']='pbmc3k'
# create a merged scanpy object and add in the scanorama
adata_merged = alldata['ctrl'].concatenate(alldata['ref'], batch_key='sample', batch_categories=['ctrl','pbmc3k'])
embedding = np.concatenate([ad.obsm['X_scanorama'] for ad in adatas], axis=0)
adata_merged.obsm['Scanorama'] = embedding#run umap.
sc.pp.neighbors(adata_merged, n_pcs =50, use_rep = "Scanorama")
sc.tl.umap(adata_merged)computing neighbors
finished (0:00:00)
computing UMAP
finished (0:00:05)2.1 Label transfer
Using the functions from the Spatial tutorial from Scanpy we will calculate normalized cosine distances between the two datasets and tranfer labels to the celltype with the highest scores.
from sklearn.metrics.pairwise import cosine_distances
distances = 1 - cosine_distances(
adata_merged[adata_merged.obs['sample'] == "pbmc3k"].obsm["Scanorama"],
adata_merged[adata_merged.obs['sample'] == "ctrl"].obsm["Scanorama"],
)
def label_transfer(dist, labels, index):
lab = pd.get_dummies(labels)
class_prob = lab.to_numpy().T @ dist
norm = np.linalg.norm(class_prob, 2, axis=0)
class_prob = class_prob / norm
class_prob = (class_prob.T - class_prob.min(1)) / class_prob.ptp(1)
# convert to df
cp_df = pd.DataFrame(
class_prob, columns=lab.columns
)
cp_df.index = index
# classify as max score
m = cp_df.idxmax(axis=1)
return m
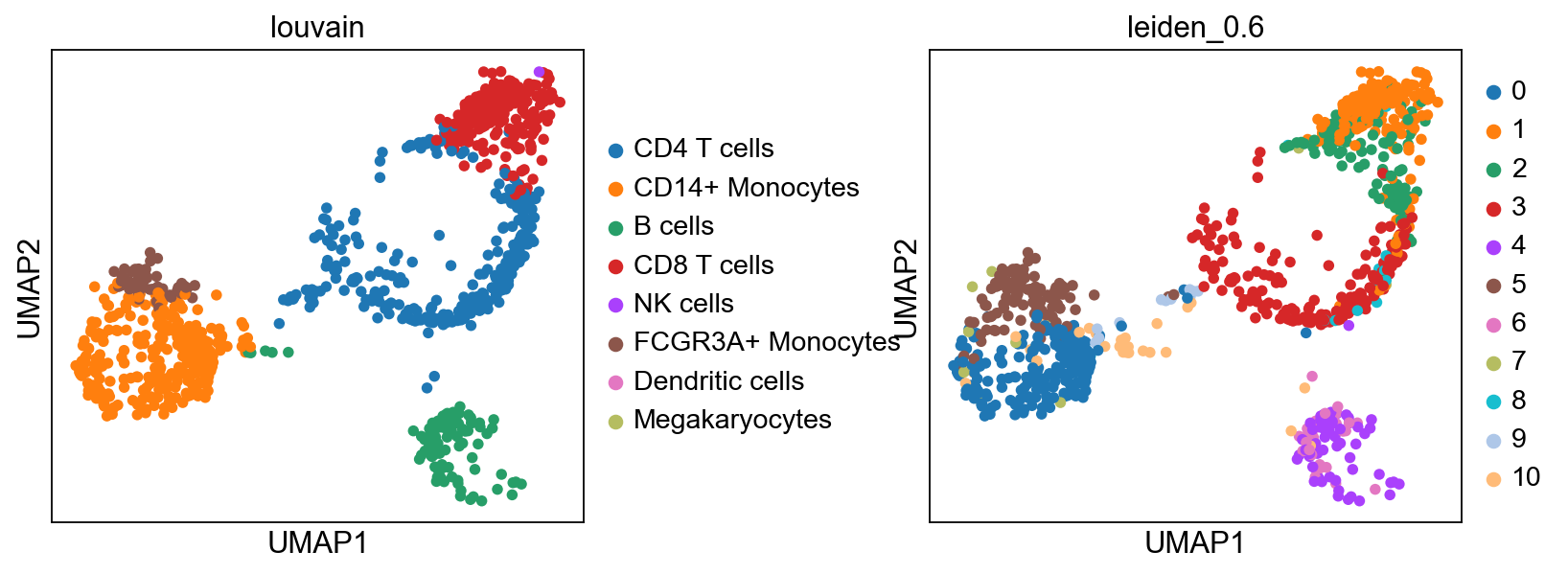
class_def = label_transfer(distances, adata_ref.obs.louvain, adata.obs.index)
# add to obs section of the original object
adata.obs['label_trans'] = class_def
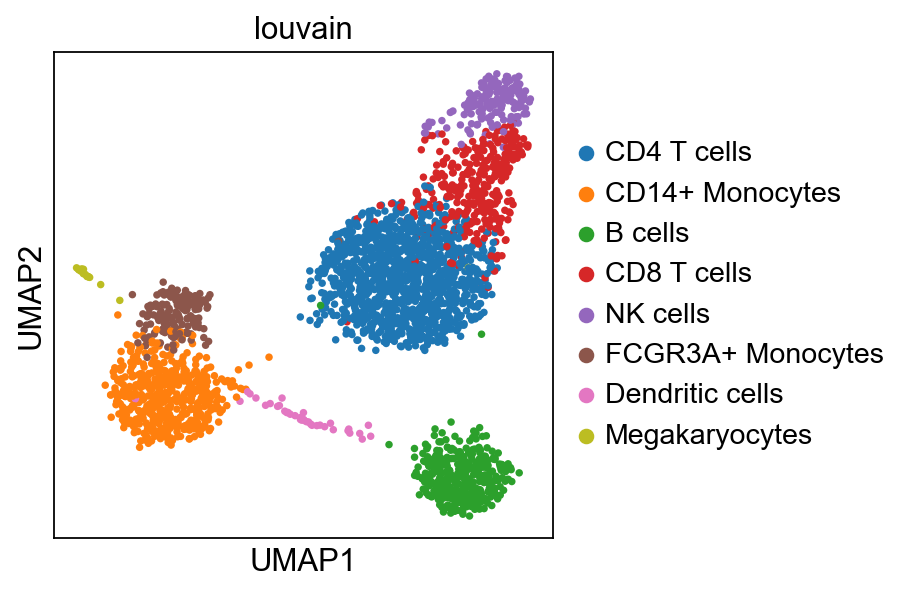
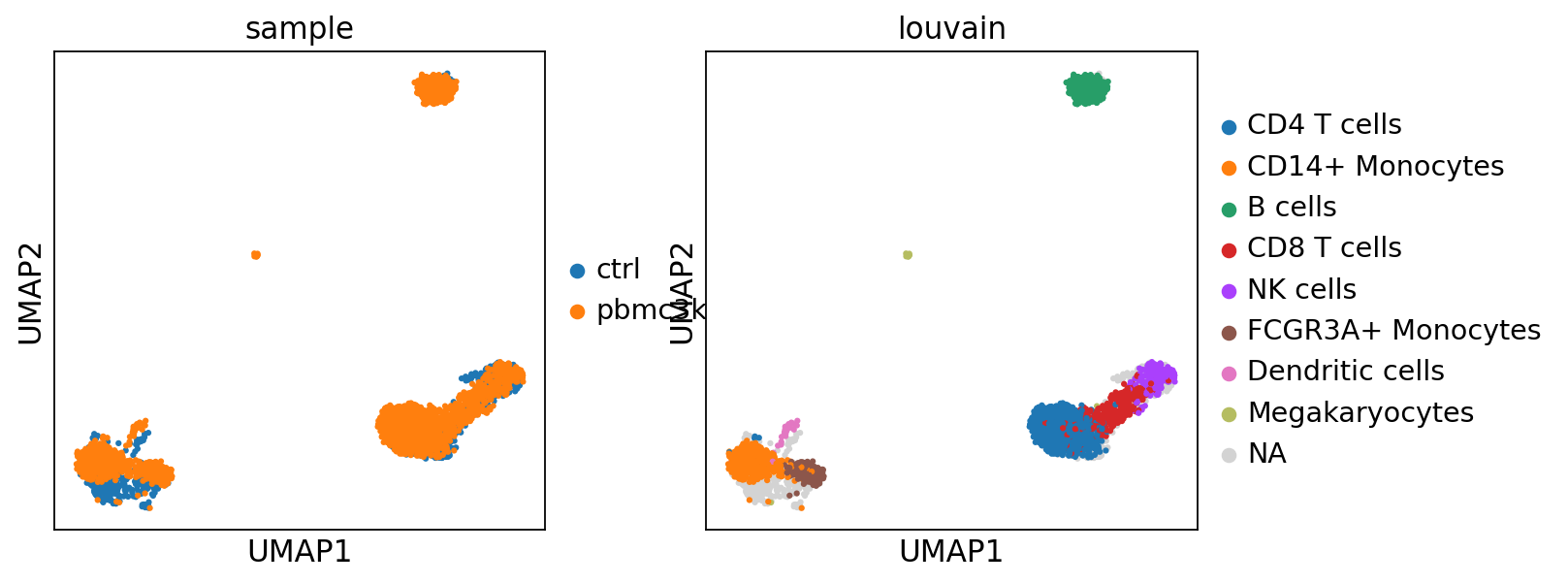
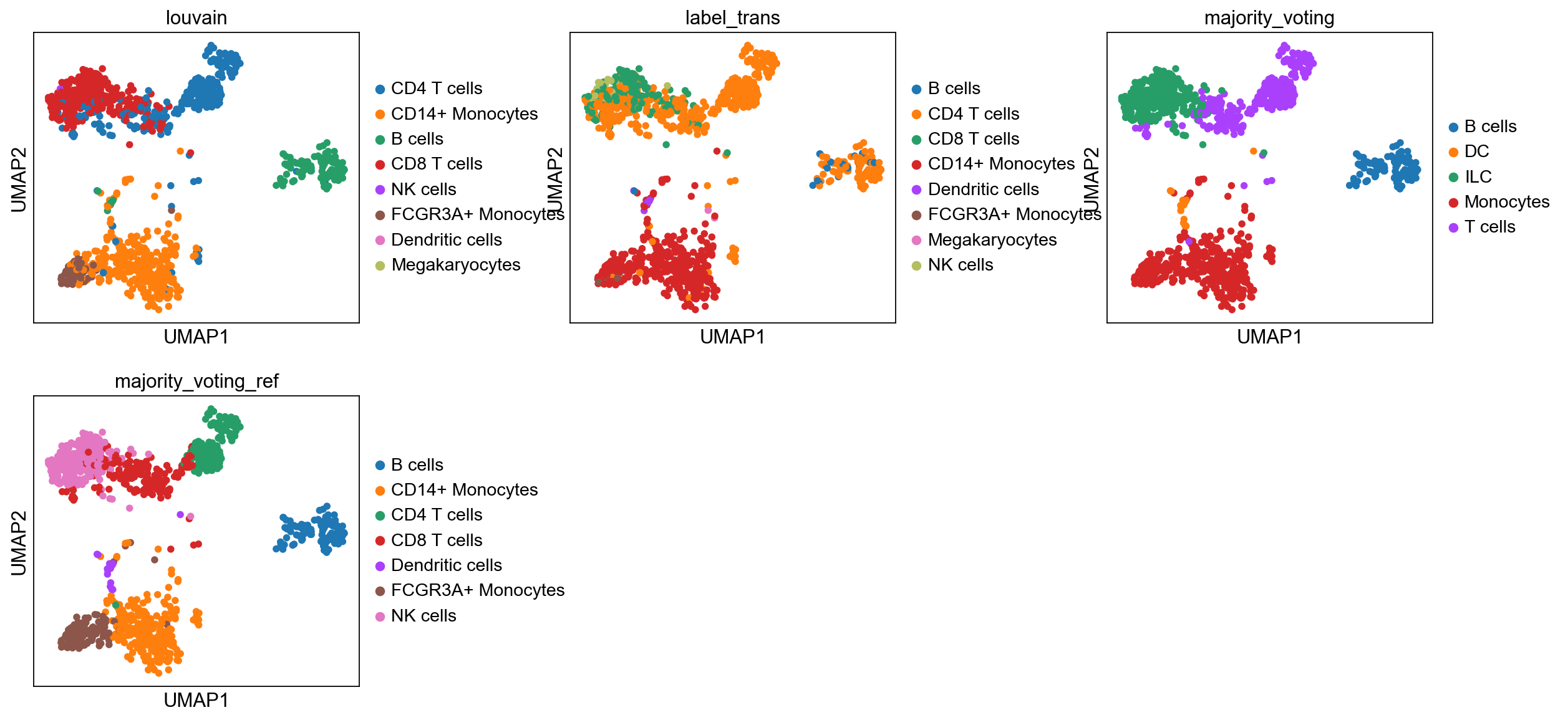
sc.pl.umap(adata, color="label_trans")# add to merged object.
adata_merged.obs["label_trans"] = pd.concat(
[class_def, adata_ref.obs["louvain"]], axis=0
).tolist()
sc.pl.umap(adata_merged, color=["sample","louvain",'label_trans'])
#plot only ctrl cells.
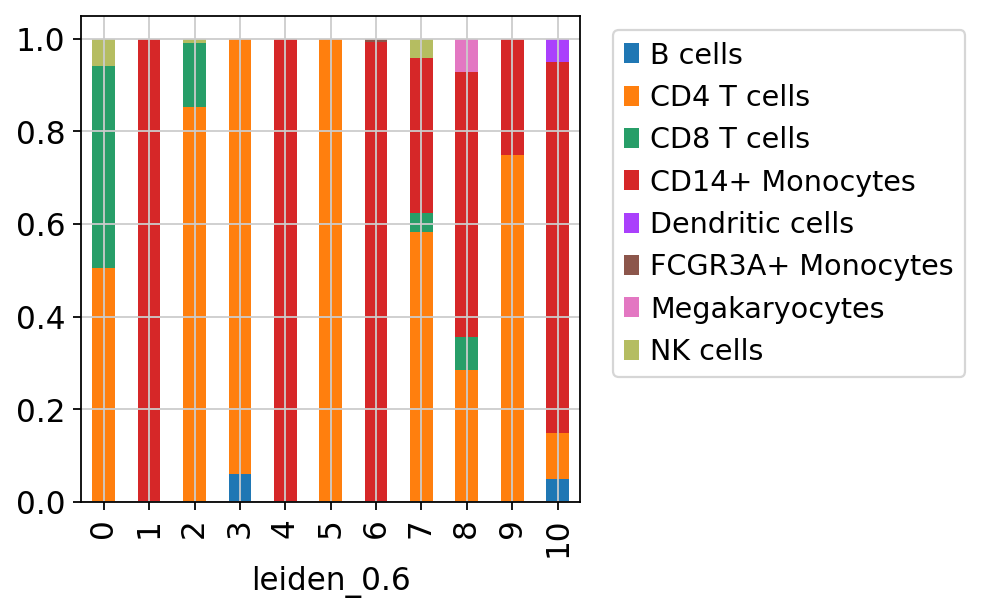
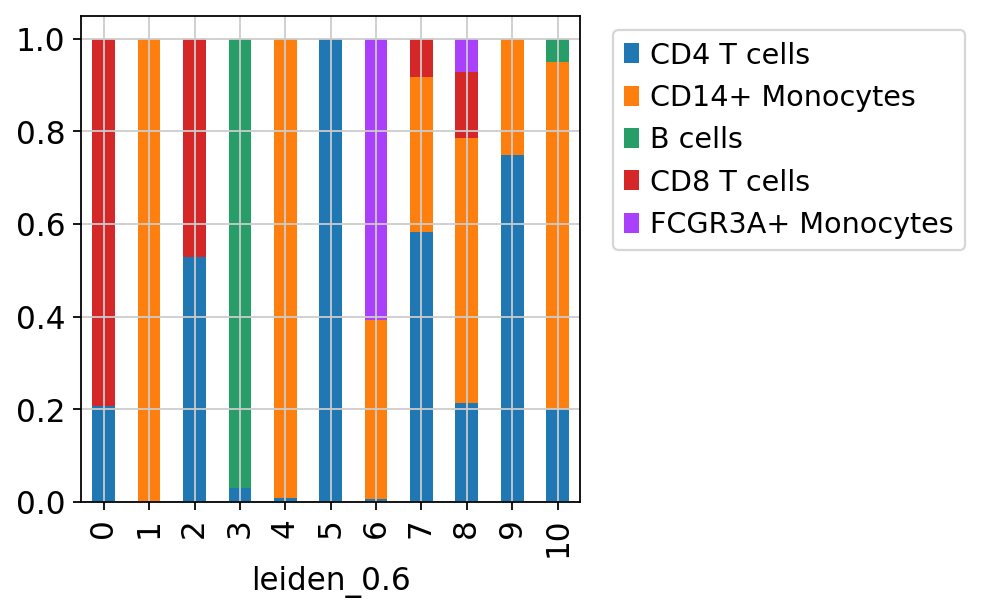
sc.pl.umap(adata_merged[adata_merged.obs['sample']=='ctrl'], color='label_trans')Now plot how many cells of each celltypes can be found in each cluster.
3 Ingest
Another method for celltype prediction is Ingest, for more information, please look at https://scanpy-tutorials.readthedocs.io/en/latest/integrating-data-using-ingest.html
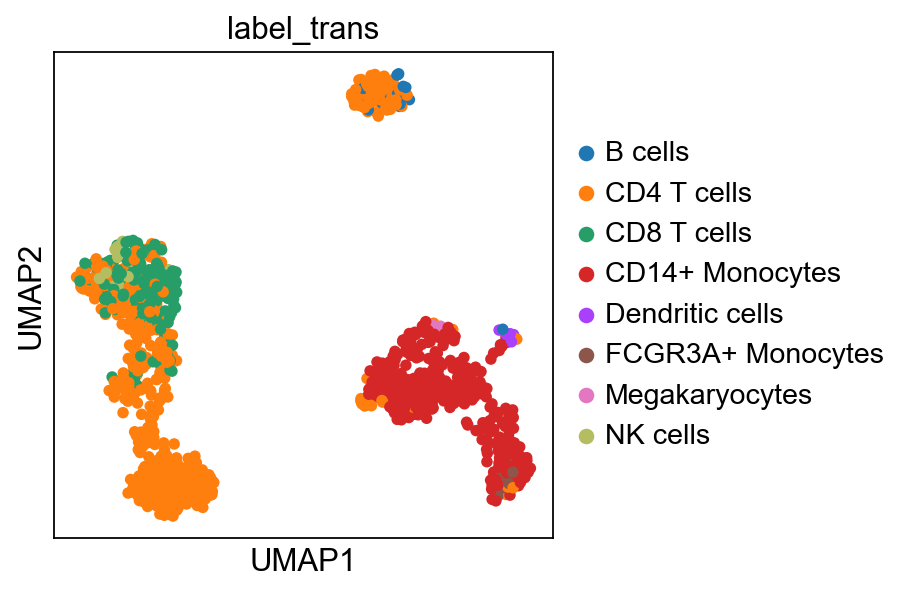
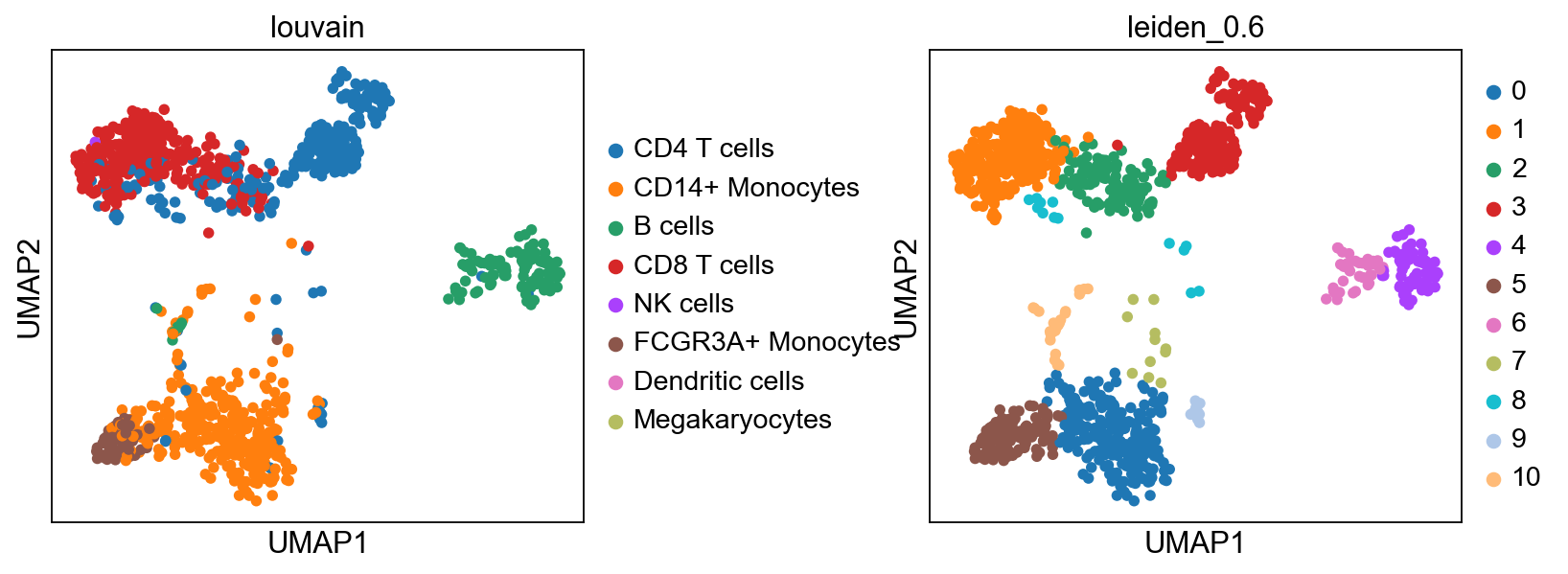
sc.tl.ingest(adata, adata_ref, obs='louvain')
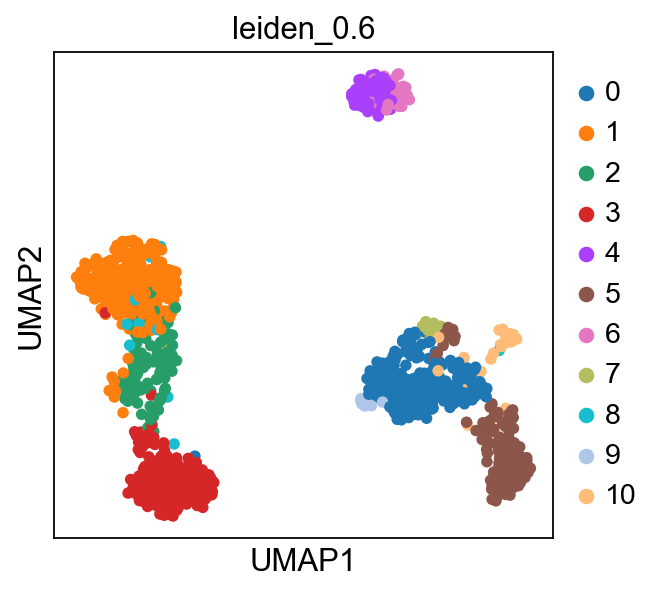
sc.pl.umap(adata, color=['louvain','leiden_0.6'], wspace=0.5)running ingest
finished (0:00:22)As you can see, ingest has created a new umap for us, so to get consistent plotting, lets revert back to the old one for further plotting:
adata.obsm["X_umap"] = adata.obsm["X_umap_uncorr"]
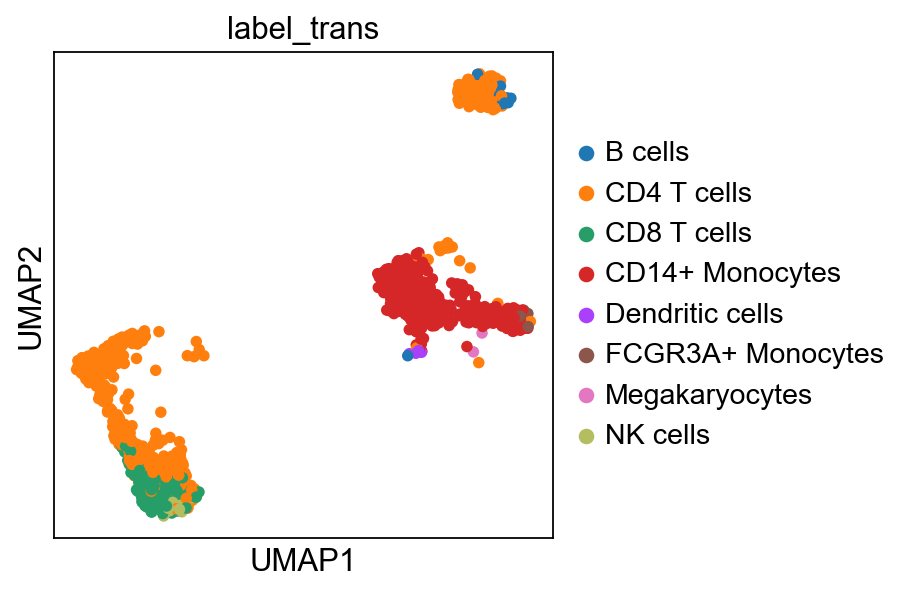
sc.pl.umap(adata, color=['louvain','leiden_0.6'], wspace=0.5)Now plot how many cells of each celltypes can be found in each cluster.
4 Celltypist
Celltypist provides pretrained models for classification for many different human tissues and celltypes. Here, we are following the steps of this tutorial, with some adaptations for this dataset. So please check out the tutorial for more detail.
import celltypist
from celltypist import models
# there are many different models, we will only download 2 of them for now.
models.download_models(force_update = False, model = 'Immune_All_Low.pkl')
models.download_models(force_update = False, model = 'Immune_All_High.pkl')Now select the model you want to use and show the info:
model = models.Model.load(model = 'Immune_All_High.pkl')
modelCellTypist model with 32 cell types and 6639 features
date: 2022-07-16 08:53:00.959521
details: immune populations combined from 20 tissues of 18 studies
source: https://doi.org/10.1126/science.abl5197
version: v2
cell types: B cells, B-cell lineage, ..., pDC precursor
features: A1BG, A2M, ..., ZYXTo infer celltype labels to our cells, we first need to convert back to the full matrix. OBS! For celltypist we want to have log1p normalised expression to 10,000 counts per cell. Which we already have in adata.raw.X, check by summing up the data, it should sum to 10K.
adata = adata.raw.to_adata()
adata.X.expm1().sum(axis = 1)[:10]matrix([[10000.],
[10000.],
[10000.],
[10000.],
[10000.],
[10000.],
[10000.],
[10000.],
[10000.],
[10000.]])predictions = celltypist.annotate(adata, model = 'Immune_All_High.pkl', majority_voting = True)
predictions.predicted_labelsrunning Leiden clustering
finished (0:00:00)| predicted_labels | over_clustering | majority_voting | |
|---|---|---|---|
| AGGTCATGTGCGAACA-13-5 | T cells | 15 | T cells |
| CCTATCGGTCCCTCAT-13-5 | ILC | 18 | ILC |
| TCCTCCCTCGTTCATT-13-5 | HSC/MPP | 23 | ILC |
| CAACCAATCATCTATC-13-5 | ILC | 18 | ILC |
| TACGGTATCGGATTAC-13-5 | T cells | 5 | T cells |
| ... | ... | ... | ... |
| TCCACCATCATAGCAC-13-5 | T cells | 15 | T cells |
| GAGTTACAGTGAGTGC-13-5 | T cells | 11 | T cells |
| ATCATTCAGGCTCACC-13-5 | Monocytes | 20 | Monocytes |
| AGCCACGCAACCCTAA-13-5 | T cells | 0 | T cells |
| CTACCTGGTCAGGAGT-13-5 | ILC | 32 | ILC |
1154 rows × 3 columns
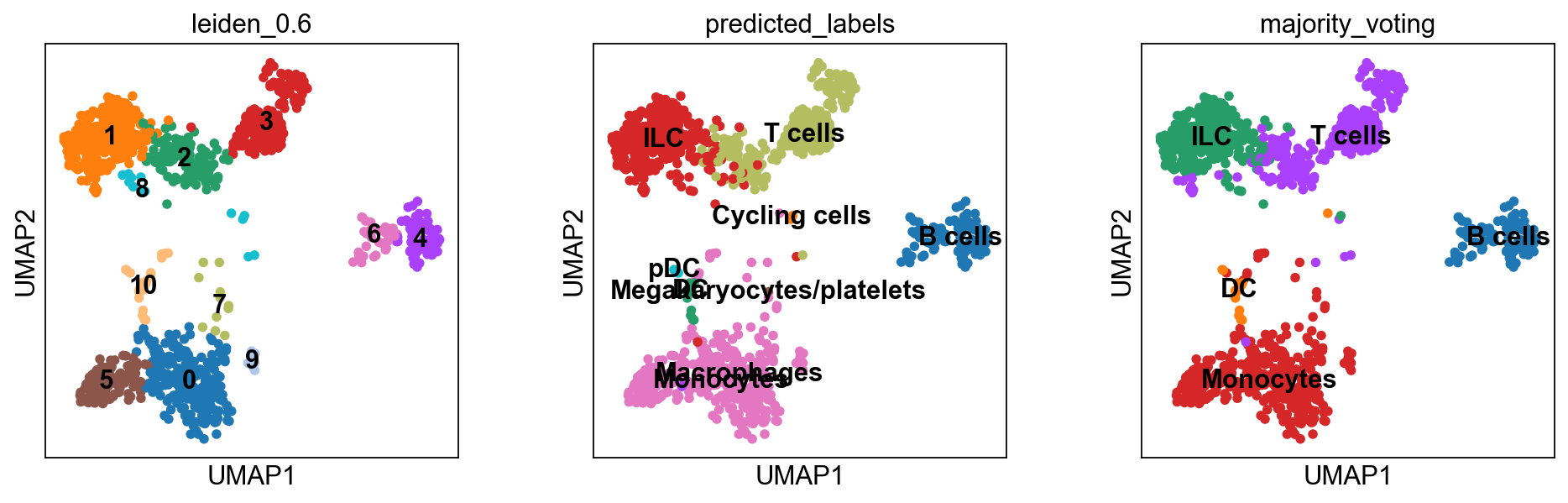
The first column predicted_labels is the predictions made for each individual cell, while majority_voting is done for local subclusters, the clustering identities are in column over_clustering.
Now we convert the predictions to an anndata object.
adata = predictions.to_adata()
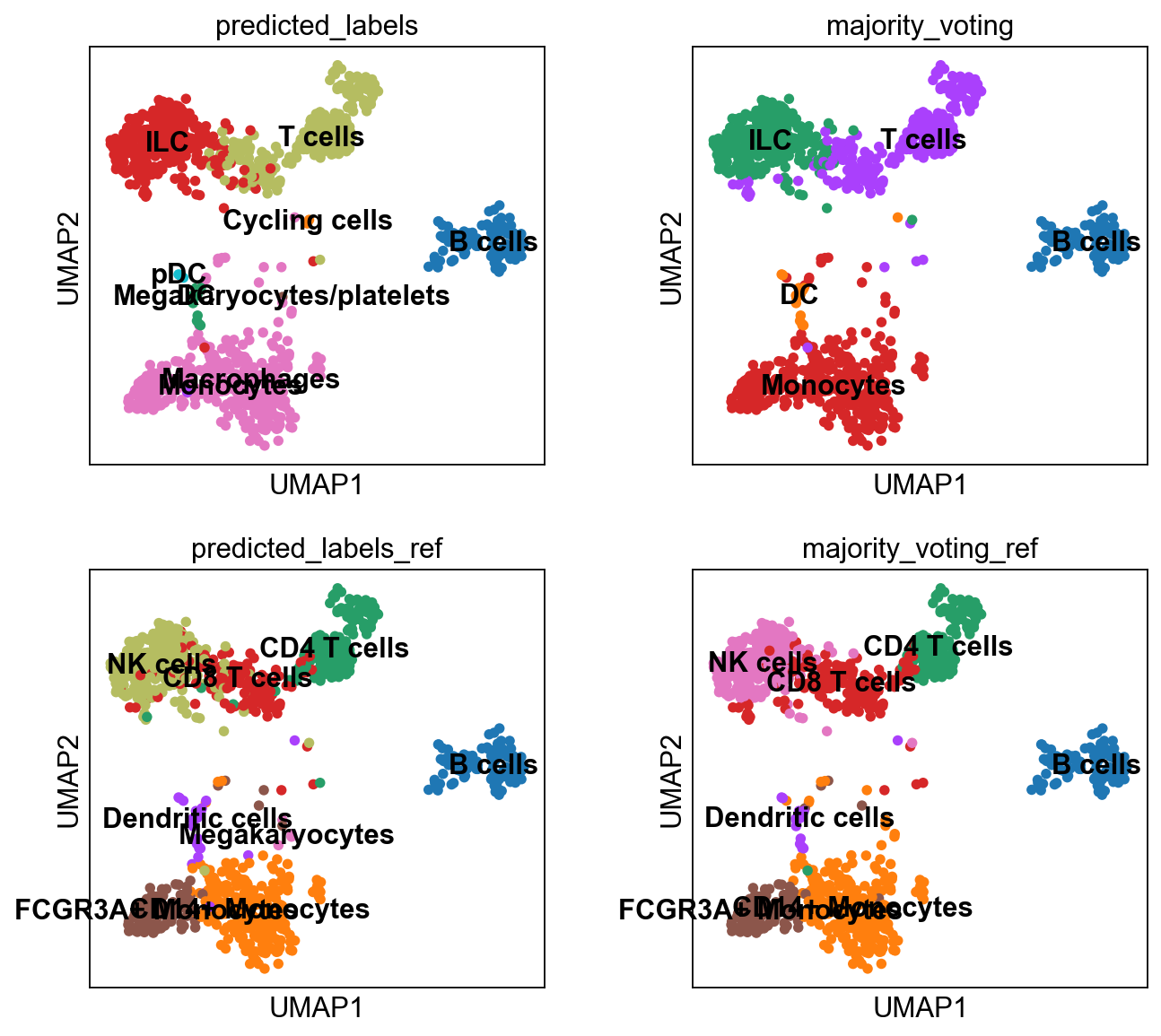
sc.pl.umap(adata, color = ['leiden_0.6', 'predicted_labels', 'majority_voting'], legend_loc = 'on data')Rerun predictions with Celltypist, but use another model, for instance Immune_All_High.pkl, or any other model you find relevant, you can find a list of models here. How do the results differ for you?
4.1 Celltypist custom model
We can also train our own model on any reference data that we want to use. In this case we will use the pbmc data in adata_ref to train a model.
Celltypist requires the data to be in the format of log1p normalised expression to 10,000 counts per cell, we can check if that is the case for the object we have:
adata_ref.raw.X.expm1().sum(axis = 1)[:10]matrix([[2419.],
[4903.],
[3147.],
[2639.],
[ 980.],
[2163.],
[2175.],
[2260.],
[1275.],
[1103.]], dtype=float32)These should all sum up to 10K, which is not the case, probably since some genes were removed after normalizing. Wo we will have to start from the raw counts of that dataset instead. Before we selected the data pbmc3k_processed, but now we will instead use pbmc3k.
adata_ref2 = sc.datasets.pbmc3k()
adata_ref2AnnData object with n_obs × n_vars = 2700 × 32738
var: 'gene_ids'This data is not annotated, so we will have to match the indices from the filtered and processed object. And add in the metadata with annotations.
adata_ref2 = adata_ref2[adata_ref.obs_names,:]
adata_ref2.obs = adata_ref.obs
adata_ref2AnnData object with n_obs × n_vars = 2638 × 32738
obs: 'n_genes', 'percent_mito', 'n_counts', 'louvain', 'sample'
var: 'gene_ids'Now we can normalize the matrix:
sc.pp.normalize_total(adata_ref2, target_sum = 1e4)
sc.pp.log1p(adata_ref2)
# check the sums again
adata_ref2.X.expm1().sum(axis = 1)[:10]normalizing counts per cell
finished (0:00:00)matrix([[10000. ],
[10000. ],
[10000. ],
[ 9999.998],
[ 9999.998],
[10000. ],
[ 9999.999],
[10000. ],
[10000.001],
[10000. ]], dtype=float32)And finally train the model.
new_model = celltypist.train(adata_ref2, labels = 'louvain', n_jobs = 10, feature_selection = True)Now we can run predictions on our data
predictions2 = celltypist.annotate(adata, model = new_model, majority_voting = True)running Leiden clustering
finished (0:00:00)Instead of converting the predictions to anndata we will just add another column in the adata.obs with these new predictions since the column names from the previous celltypist runs with clash.
adata.obs["predicted_labels_ref"] = predictions2.predicted_labels["predicted_labels"]
adata.obs["majority_voting_ref"] = predictions2.predicted_labels["majority_voting"]5 Compare results
The predictions from ingest is stored in the column ‘louvain’ while we named the label transfer with scanorama as ‘predicted’
sc.pl.umap(adata, color=['louvain','label_trans','majority_voting', 'majority_voting_ref'], wspace=0.5, ncols=3)As you can see, the main celltypes are generally the same, but there are clearly differences, especially with regards to the cells predicted as either ILC/NK/CD8 T-cells.
The only way to make sure which method you trust is to look at what genes the different celltypes express and use your biological knowledge to make decisions.
6 Gene set analysis
Another way of predicting celltypes is to use the differentially expressed genes per cluster and compare to lists of known cell marker genes. This requires a list of genes that you trust and that is relevant for the tissue you are working on.
You can either run it with a marker list from the ontology or a list of your choice as in the example below.
path_file = 'data/human_cell_markers.txt'
if not os.path.exists(path_file):
file_url = os.path.join(path_data, "misc/human_cell_markers.txt")
subprocess.call(["curl", "-u", curl_upass, "-o", path_file, file_url ])df = pd.read_table(path_file)
df
print(df.shape)(2868, 15)# Filter for number of genes per celltype
df['nG'] = df.geneSymbol.str.split(",").str.len()
df = df[df['nG'] > 5]
df = df[df['nG'] < 100]
d = df[df['cancerType'] == "Normal"]
print(df.shape)
# convert to dict.
df.index = df.cellName
gene_dict = df.geneSymbol.str.split(",").to_dict()(445, 16)# run differential expression per cluster
sc.tl.rank_genes_groups(adata, 'leiden_0.6', method='wilcoxon', key_added = "wilcoxon")ranking genes
finished (0:00:01)# do gene set overlap to the groups in the gene list and top 300 DEGs.
import gseapy
gsea_res = dict()
pred = dict()
for cl in adata.obs['leiden_0.6'].cat.categories.tolist():
print(cl)
glist = sc.get.rank_genes_groups_df(adata, group=cl, key='wilcoxon')[
'names'].squeeze().str.strip().tolist()
enr_res = gseapy.enrichr(gene_list=glist[:300],
organism='Human',
gene_sets=gene_dict,
background=adata.shape[1],
cutoff=1)
if enr_res.results.shape[0] == 0:
pred[cl] = "Unass"
else:
enr_res.results.sort_values(
by="P-value", axis=0, ascending=True, inplace=True)
print(enr_res.results.head(2))
gsea_res[cl] = enr_res
pred[cl] = enr_res.results["Term"][0]0
Gene_set Term Overlap P-value Adjusted P-value \
3 gs_ind_0 Parietal progenitor cell 1/7 0.103024 0.255621
0 gs_ind_0 Effector T cell 1/13 0.182865 0.255621
Odds Ratio Combined Score Genes
3 14.764993 33.557802 ANXA1
0 7.675392 13.040543 IL2RB
1
Gene_set Term Overlap P-value Adjusted P-value \
1 gs_ind_0 Cancer stem-like cell 1/6 0.088981 0.19465
20 gs_ind_0 Spermatogonial stem cell 1/6 0.088981 0.19465
Odds Ratio Combined Score Genes
1 17.450448 42.218477 ANPEP
20 17.450448 42.218477 BCL6
2
Gene_set Term Overlap P-value Adjusted P-value \
3 gs_ind_0 Monocyte 1/7 0.103024 0.206048
4 gs_ind_0 Parietal progenitor cell 1/7 0.103024 0.206048
Odds Ratio Combined Score Genes
3 14.764993 33.557802 CD52
4 14.764993 33.557802 ANXA1
3
Gene_set Term Overlap P-value Adjusted P-value Odds Ratio \
0 gs_ind_0 B cell 1/6 0.088981 0.116851 17.450448
3 gs_ind_0 Monocyte 1/7 0.103024 0.116851 14.764993
Combined Score Genes
0 42.218477 CD19
3 33.557802 CD52
4
Gene_set Term Overlap P-value Adjusted P-value \
0 gs_ind_0 Cancer stem-like cell 1/6 0.088981 0.218659
5 gs_ind_0 Macrophage 1/6 0.088981 0.218659
Odds Ratio Combined Score Genes
0 17.450448 42.218477 ANPEP
5 17.450448 42.218477 AIF1
5
Gene_set Term Overlap P-value Adjusted P-value \
3 gs_ind_0 Effector memory T cell 1/7 0.103024 0.215807
5 gs_ind_0 Monocyte 1/7 0.103024 0.215807
Odds Ratio Combined Score Genes
3 14.764993 33.557802 IL7R
5 14.764993 33.557802 CD52
6
Gene_set Term Overlap P-value \
1 gs_ind_0 Macrophage 1/6 0.088981
2 gs_ind_0 Monocyte derived dendritic cell 1/8 0.116851
Adjusted P-value Odds Ratio Combined Score Genes
1 0.175277 17.450448 42.218477 AIF1
2 0.175277 12.795659 27.470422 ITGAX
7
Gene_set Term Overlap P-value Adjusted P-value \
1 gs_ind_0 Circulating fetal cell 1/40 0.463035 0.712041
0 gs_ind_0 AXL+SIGLEC6+ dendritic cell 1/80 0.712041 0.712041
Odds Ratio Combined Score Genes
1 2.425498 1.867518 ACTB
0 1.202602 0.408427 ACPP
8
Gene_set Term Overlap P-value \
0 gs_ind_0 CD1C+_A dendritic cell 1/31 0.382330
1 gs_ind_0 Effector CD8+ memory T (Tem) cell 1/83 0.725203
Adjusted P-value Odds Ratio Combined Score Genes
0 0.725203 3.142697 3.021616 ADAM8
1 0.725203 1.158689 0.372291 ADAM8
9
Gene_set Term Overlap P-value Adjusted P-value \
2 gs_ind_0 Lake et al.Science.In4 1/6 0.088981 0.212585
1 gs_ind_0 Immune cell 1/7 0.103024 0.212585
Odds Ratio Combined Score Genes
2 17.450448 42.218477 ADAMTS2
1 14.764993 33.557802 CD14
10
Gene_set Term Overlap P-value Adjusted P-value \
2 gs_ind_0 PROM1Low progenitor cell 1/7 0.103024 0.215807
0 gs_ind_0 Memory B cell 1/10 0.143872 0.215807
Odds Ratio Combined Score Genes
2 14.764993 33.557802 ALCAM
0 10.100782 19.583741 RBPJ # prediction per cluster
pred{'0': 'Effector T cell',
'1': 'CD16+ dendritic cell',
'2': 'CD8+ T cell',
'3': 'B cell',
'4': 'Cancer stem-like cell',
'5': 'CD4+ T cell',
'6': 'Conventional dendritic cell',
'7': 'AXL+SIGLEC6+ dendritic cell',
'8': 'CD1C+_A dendritic cell',
'9': 'Circulating fetal cell',
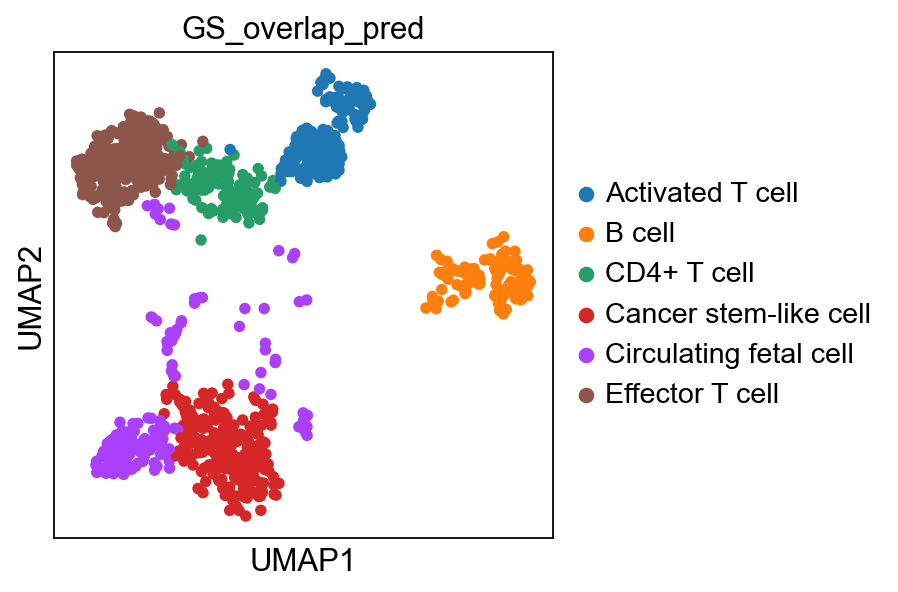
'10': 'Memory B cell'}prediction = [pred[x] for x in adata.obs['leiden_0.6']]
adata.obs["GS_overlap_pred"] = prediction
sc.pl.umap(adata, color='GS_overlap_pred')As you can see, it agrees to some extent with the predictions from the methods above, but there are clear differences, which do you think looks better?
7 Save data
We can finally save the object for use in future steps.
adata.write_h5ad('data/covid/results/scanpy_covid_qc_dr_int_cl_ct-ctrl13.h5ad')8 Session info
Click here
sc.logging.print_versions()-----
anndata 0.10.8
scanpy 1.10.3
-----
PIL 11.1.0
annoy NA
array_api_compat 1.10.0
asttokens NA
brotli 1.1.0
celltypist 1.6.3
certifi 2024.12.14
cffi 1.17.1
charset_normalizer 3.4.1
colorama 0.4.6
comm 0.2.2
cycler 0.12.1
cython_runtime NA
dateutil 2.9.0.post0
debugpy 1.8.12
decorator 5.1.1
exceptiongroup 1.2.2
executing 2.1.0
fbpca NA
gseapy 1.1.3
h5py 3.12.1
idna 3.10
igraph 0.11.6
intervaltree NA
ipykernel 6.29.5
jedi 0.19.2
joblib 1.4.2
kiwisolver 1.4.7
legacy_api_wrap NA
leidenalg 0.10.2
llvmlite 0.43.0
matplotlib 3.9.2
matplotlib_inline 0.1.7
mpl_toolkits NA
natsort 8.4.0
numba 0.60.0
numpy 1.26.4
packaging 24.2
pandas 1.5.3
parso 0.8.4
patsy 1.0.1
pickleshare 0.7.5
platformdirs 4.3.6
prompt_toolkit 3.0.50
psutil 6.1.1
pure_eval 0.2.3
pycparser 2.22
pydev_ipython NA
pydevconsole NA
pydevd 3.2.3
pydevd_file_utils NA
pydevd_plugins NA
pydevd_tracing NA
pygments 2.19.1
pynndescent 0.5.13
pyparsing 3.2.1
pytz 2024.2
requests 2.32.3
scanorama 1.7.4
scipy 1.14.1
session_info 1.0.0
six 1.17.0
sklearn 1.6.1
socks 1.7.1
sortedcontainers 2.4.0
sparse 0.15.5
stack_data 0.6.3
statsmodels 0.14.4
texttable 1.7.0
threadpoolctl 3.5.0
torch 2.5.1.post207
torchgen NA
tornado 6.4.2
tqdm 4.67.1
traitlets 5.14.3
typing_extensions NA
umap 0.5.7
urllib3 2.3.0
wcwidth 0.2.13
yaml 6.0.2
zmq 26.2.0
zoneinfo NA
zstandard 0.23.0
-----
IPython 8.31.0
jupyter_client 8.6.3
jupyter_core 5.7.2
-----
Python 3.10.16 | packaged by conda-forge | (main, Dec 5 2024, 14:16:10) [GCC 13.3.0]
Linux-6.10.14-linuxkit-x86_64-with-glibc2.35
-----
Session information updated at 2025-02-27 15:24