Genetic diversity
What determines diversity levels?
The usual questions:
What evolutionary forces maintain genetic diversity in natural populations? How do diversity levels relate to census population sizes…? Do low levels of diversity limit adaptation to selective pressures?
Leffler et al. (2012)
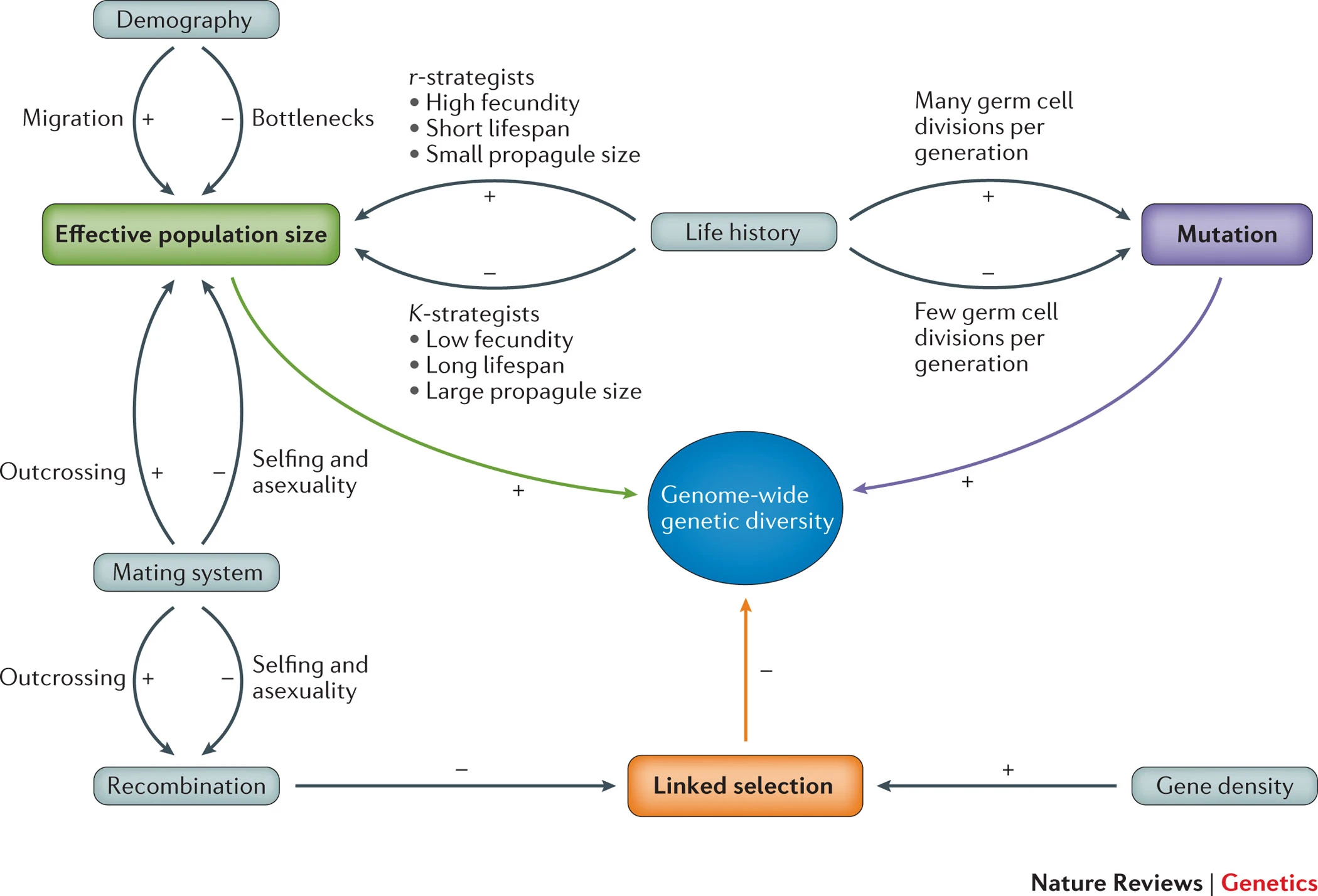
After allozyme era, the study of genetic diversity was largely neglected due to lack of genome-wide data, but with advent of population genomics becoming a hot topic again.
Ellegren & Galtier (2016)
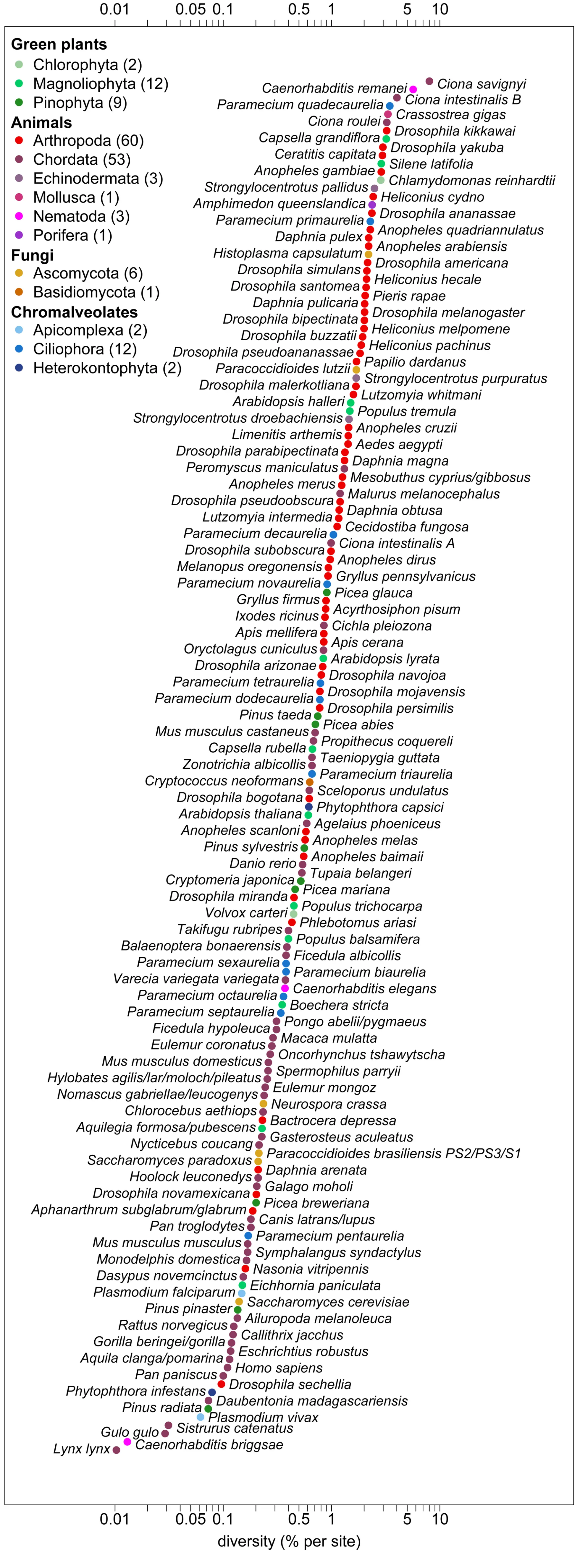
Lewontin’s paradox: genetic diversity range smaller than variation among species in population size
Factors that influence genetic diversity
Genetic drift
Reduces diversity at loss \propto \frac{1}{N}
Selection
Adaptive selection decreases variation, more so if acting on new mutations compared to standing variation.
Balancing selection may increase variation.
Recombination
Low recombination rates lead to less “reshuffling” of variation and hence lower diversity.
Why we measure patterns of genetic variation
Genetic variation patterns are informative of evolutionary and demographic processes. We often use summary statistics to describe the patterns, and to estimate parameters such as effective population size and mutation rate from genetic variation data (\theta = 4N_e\mu)
Often critical first step of analysis, such as
- exploratory study
- test of an evolutionary hypothesis
- training of machine-learning models
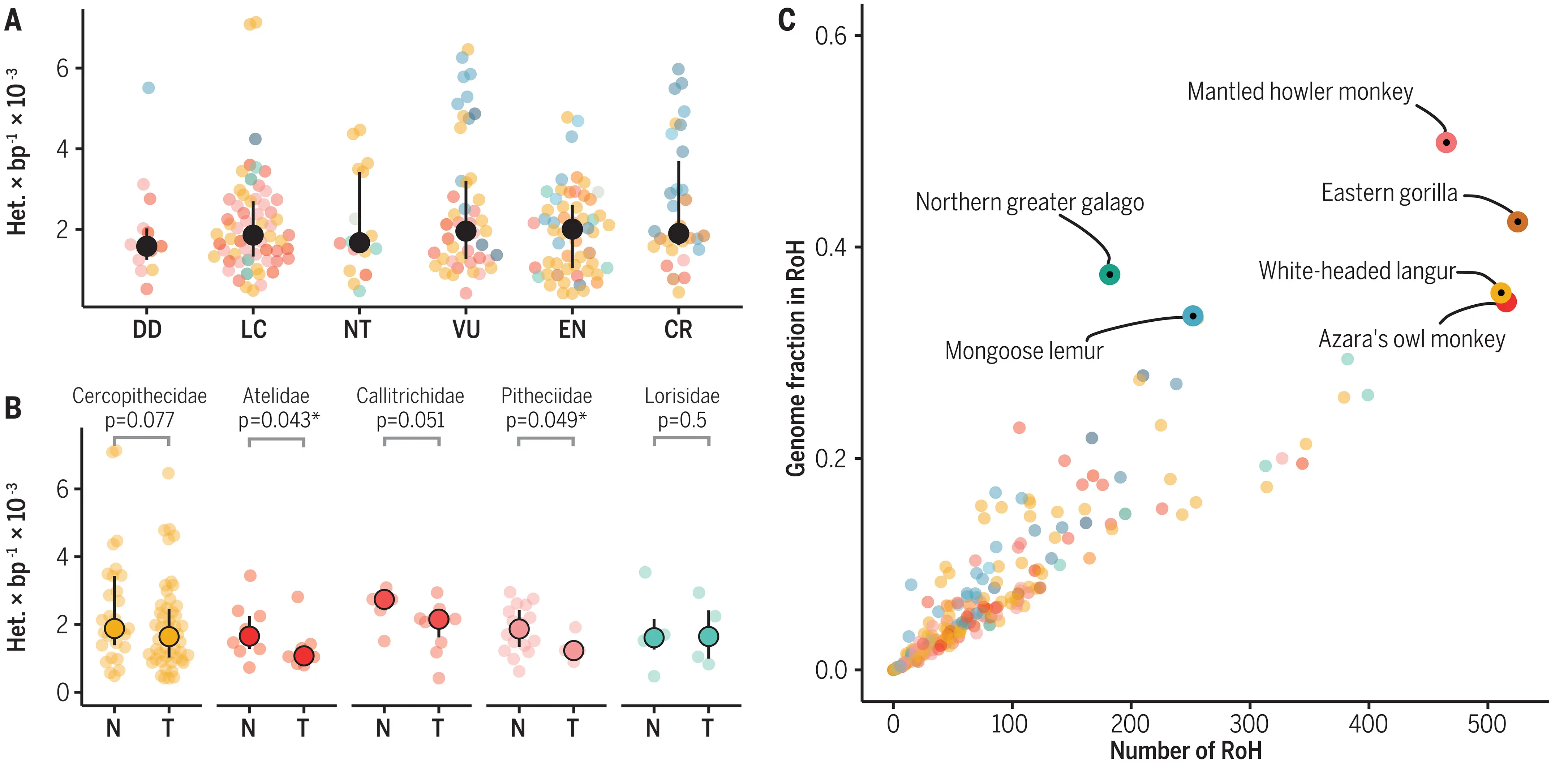
Genetic diversity in conservation biology
…no global relationship between numerically coded IUCN extinction risk categories and estimated heterozygosity…
Low genetic diversity symptom of past genetic drift inbreeding (higher levels of homozygosity), caused by low N_e
García-Dorado & Caballero (2021)
However: if population decline is rapid, may be too little time for inbreeding to occur \Rightarrow genetic diversity within species not necessarily aligned to extinction risk
Lewis (2023)
Measuring genetic diversity
Nucleotide diversity
sample 0: 00100
sample 1: 00001
sample 2: 01010
sample 3: 10010Diversity: for each site, count and sum differences between all (unique) pairs of samples, and divide by unique pairs. For n samples, there are n \choose 2 such pairs.
Example: for site 0, start comparing samples 0-1 (0 diffs), samples 0-2 (0), samples 0-3 (1), samples 1-2 (0) and so on. Call these differences k_{ij}. Then
\pi = \frac{\sum_{i<j}k_{ij}}{n \choose 2}
Watterson’s \theta_W
sample 0: 00100
sample 1: 00001
sample 2: 01010
sample 3: 10010Alternative measure of diversity: simply count the number of segregating sites (S). However, must correct for the number of samples n as we expect that more samples \Rightarrow more sites
\theta_W = \frac{S}{a} = \frac{S}{\sum_{i=1}^{n-1}\frac{1}{i}}
Important: under neutrality, \theta = E(\pi) = E(\theta_W). Difference between two the basis for Tajima’s D that is a test for selection
Calculating diversity measures - \pi and \theta_W
sample 0: 00100
sample 1: 00001
sample 2: 01010
sample 3: 10010from trees import ts_small_mut as ts
# Calculate correction factor a for Watterson's
# theta: the larger the sample size, the more
# segregating sites we expect to see
a = sum([1/i for i in range(1, ts.num_samples)])
pi = ts.diversity()
thetaW = ts.num_sites / a / ts.sequence_length
print(f"Diversity: {pi:.6f}",
f"Watterson's theta: {thetaW:.6f}",
f"Sequence length: {ts.sequence_length:.0f}",
sep="\n")Diversity: 0.000267
Watterson's theta: 0.000273
Sequence length: 10000Divergence - dXY
Sample 0: 00100
Sample 1: 00001
Sample 2: 01010
Sample 3: 10010Divergence: for each site, count and sum differences between all pairs of samples between two populations
Example: for site 0, compare samples 0-2 (0 diffs), samples 0-3 (1 diff), samples 1-2 (0 diffs), samples 1-3 (1 diff), and so on. Call differences k_ij, let n_X, n_Y be sample size in populations X, Y. Then
d_{XY} = \frac{1}{n_Xn_Y}\sum_{i=1}^{n_X}\sum_{i=1}^{n_Y}k_{ij}
Divergence - dXY
Differentiation - AFD (allele frequency difference)
Sample 0: 00100
Sample 1: 00001
Sample 2: 01010
Sample 3: 10010Allele Frequency Difference (AFD) proposed as intuitive alternative to F_{\mathrm{ST}}. For each site, count the difference in allele frequency between two populations.
Example: site 0, frequency in blue is 0, in black 1/2, so difference=1/2, site 1, frequency in blue 0, in black 1/2, and so on
AFD = \frac{1}{2}\sum_{i=1}^n| (f_{i1} - f_{i2})|
where n is the number of different alleles (n=2 for biallelic SNPs), f_{ij} is the frequency of allele i in population j
Berner (2019)
Differentiation - F_{\mathrm{ST}}
Sample 0: 00100
Sample 1: 00001
Sample 2: 01010
Sample 3: 10010F_{\mathrm{ST}} is another measure of differention among subpopulations (Wright, 1931). It ranges between 0 and 1 and has the interpretation 0: no differentiation, 1: complete fixation of alternate alleles in subpopulations
Example: site 3 has F_{\mathrm{ST}}=1 as it is fixed in black, not present in blue
There are many ways to express and calculate F_{\mathrm{ST}}. Example:
F_{\mathrm{ST}} = \frac{h_{\mathrm{T}} - h_{\mathrm{S}}}{h_{\mathrm{T}}}
where h_{\mathrm{T}} is the expected heterozygosity in the total population, h_{\mathrm{S}} the average of expected heterozygosities across subpopulations. For site 3, h_{\mathrm{S}}=0, h_{\mathrm{T}}=2/3.
Caveats: strongly influenced by within subpopulation levels of variation. Therefore considered relative measure (cf d_{\mathrm{XY}}, which is an absolute measure).
Differentiation - F_{\mathrm{ST}}
Sample 0: 00100
Sample 1: 00001
Sample 2: 01010
Sample 3: 10010from trees import ts_small_mut as ts
sample_sets = [[0, 1], [2, 3]]
win = [0] + [int(x.position)+1 for x in ts.sites()]
_ = win.pop()
win = win + [ts.sequence_length]
fst = ts.Fst(sample_sets=sample_sets)
fst_sites = ts.Fst(sample_sets=sample_sets,
windows=win)
print(f"Site id: {list(range(5))}",
f"Fst per site: {fst_sites}",
f"Overall Fst: {fst:.6f}",
sep="\n")Site id: [0, 1, 2, 3, 4]
Fst per site: [0. 0. 0. 1. 0.]
Overall Fst: 0.200000Many programs treat missing data as invariant
Diversity:
\pi = \frac{\sum_{i<j}k_{ij}}{n \choose 2}
Divergence:
d_{XY} = \frac{1}{n_Xn_Y}\sum_{i=1}^{n_X}\sum_{j=1}^{n_Y}k_{ij}
Here, n is the number of samples, k_{ij} tally of allelic differences between two haplotypes within (\pi) a population or between (d_{XY}) populations
Missing data may bias diversity measures downwards
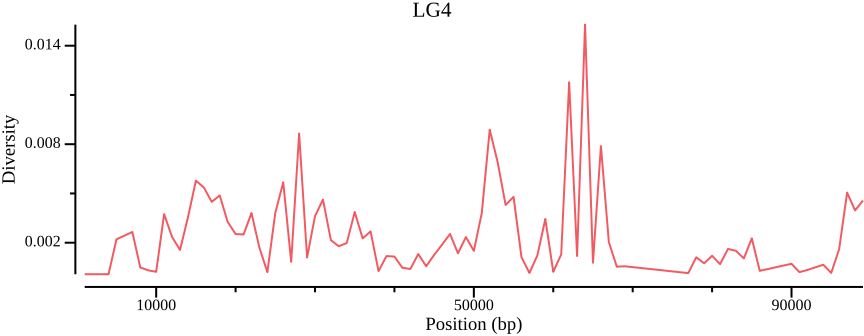
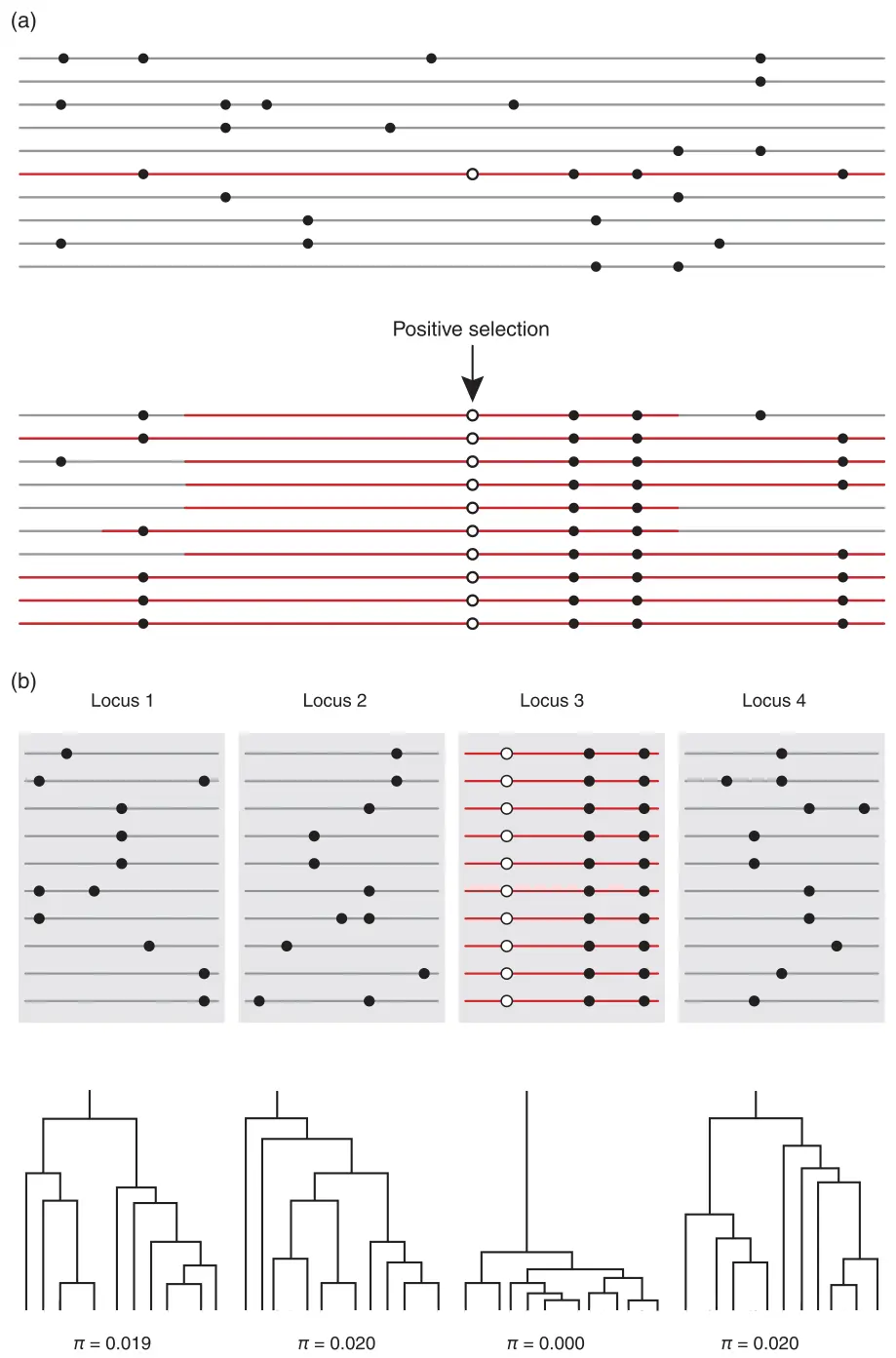
Nucleotide diversity landscapes
On genome scans
Genetic basis of adaptation and genome scans
Fundamental questions:
- How many genes are involved in the evolution of adaptive traits?
- What is the distribution of phenotypic effects among successive allelic substitutions?
- Is adaptation typically based on standing variation or new mutations?
- What is the relative importance of additive vs. nonadditive effects on adaptive trait variation?
- And what is the relative importance of structural vs. regulatory changes in phenotypic evolution?
Storz (2005), Fig 1
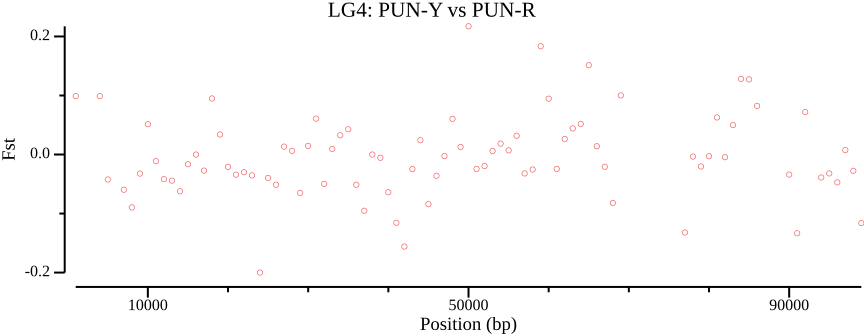
Example
vcftools --gzvcf vcf/allsites.vcf.gz --weir-fst-pop PUN-Y.txt \
--weir-fst-pop PUN-R.txt \
--fst-window-size 1000 \
--out results/out
csvtk plot line --tabs results/out.windowed.weir.fst \
-x BIN_START -y MEAN_FST \
--point-size 2 --xlab "Position (bp)" \
--ylab "Fst" --title "LG4: PUN-Y vs PUN-R" \
--width 9.0 --height 3.5 --scatter \
> results/out.windowed.weir.fst.mean.pngZ-scores can help identifying outliers
Raw data can be converted to Z-scores to highlight outliers. A Z-score is a measure of how far a data point is from the mean in terms of the number of standard deviations:
Z = \frac{X - \mu}{\sigma}
Threshold of a couple of standard deviations common.
LD decay and choice of window size
Properties of genetic variation and inferred demographic history in sampled A. millepora. Fuller et al. (2020), Figure 2. Upper left plot illustrates LD as a function of physical distance. Here, choosing a window size 20-30kb would ensure that most windows are independent.
Annotations
csvtk filter2 --tabs gff/annotation.gff --filter ' $3 == "CDS" ' |\
csvtk mutate2 --tabs -H -e '$4 - 12000000' -w 0 |\
csvtk mutate2 --tabs -H -e '$5 - 12000000' -w 0 |\
csvtk cut --tabs --fields 1,10,11 | bedtools sort | bedtools merge \
> bed/CDS.bed 2>/dev/null
head -n 3 bed/CDS.bedLG4 12032 12121
LG4 12214 12658
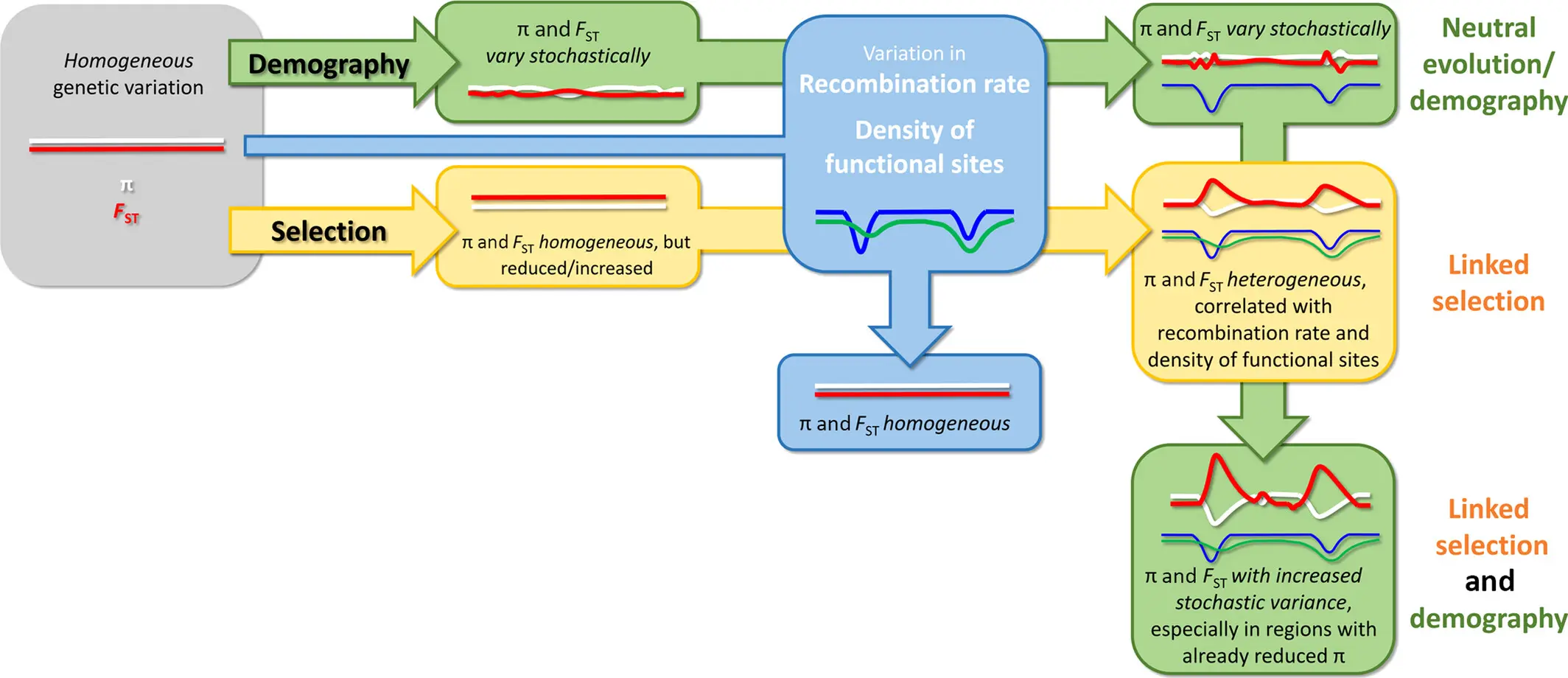
LG4 12774 12830Genome diversity and differentiation landscapes
Dissecting differentiation landscapes
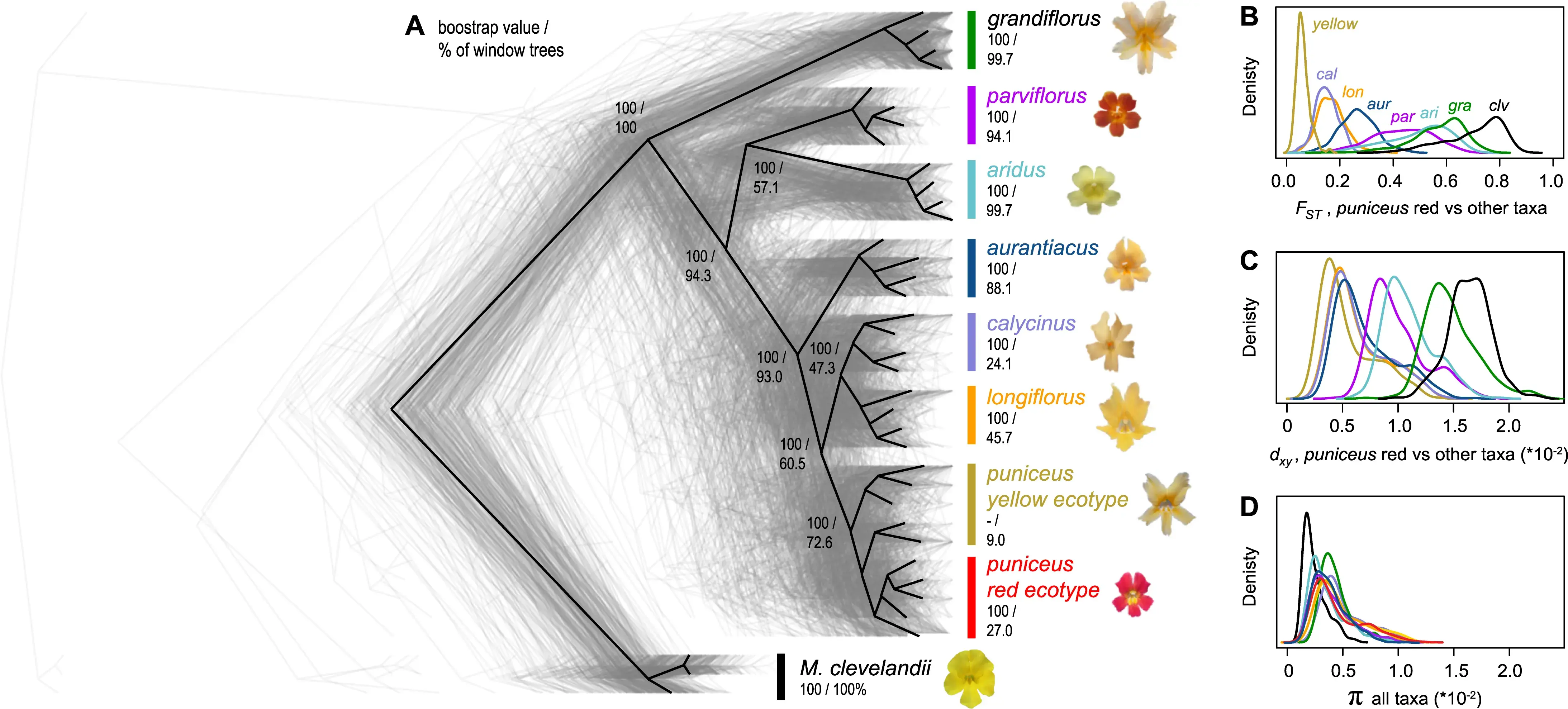
Monkeyflower genomic landscape
Exercise
Bibliography
Genetic diversity