class: center, middle, inverse, title-slide # Analysis of bulk RNA-Seq data ## Introduction To Bioinformatics Using NGS Data ### 26-Nov-2021 --- exclude: true count: false <link href="https://fonts.googleapis.com/css?family=Roboto|Source+Sans+Pro:300,400,600|Ubuntu+Mono&subset=latin-ext" rel="stylesheet"> <link rel="stylesheet" href="https://use.fontawesome.com/releases/v5.3.1/css/all.css" integrity="sha384-mzrmE5qonljUremFsqc01SB46JvROS7bZs3IO2EmfFsd15uHvIt+Y8vEf7N7fWAU" crossorigin="anonymous"> <!-- ------------ Only edit title, subtitle & author above this ------------ --> --- name: contents class: spaced # Contents * [RNA Sequencing](#intro) * [Workflow](#workflow) * [DGE Workflow](#workflow-dge) * [ReadQC](#read-qc) * [Mapping](#mapping-intro) * [Alignment QC](#alignment-qc) * [Quantification](#quantification-counts) * [Normalisation](#normalisation) * [Exploratory](#exploratory-heatmap) * [DGE](#dge-1) * [Functional analyses](#functional-analysis-1) * [Summary](#summary) * [Help](#help) --- name: intro class: spaced # What is RNA?  - The transcriptome is spatially and temporally dynamic - Data comes from functional units (coding regions) - Only a tiny fraction of the genome ??? - Central dogma of molecular biology. DNA -> RNA -> Protein. - We have gene models to explain organisation of the DNA into functional units. - Very tiny portion of the genome transcribes to RNA. - There are many types of RNA. Commonly protein coding RNA or mRNA. There are tRNA, sRNA, miRNA, siRNA, lincRNA etc. - What is the most abundant RNA? rRNA. - Why are we interested in RNA? While DNA is constant in all cells, expressed RNA varies from cell to cell and from time to time. - When we say RNA sequencing, what we sequencing?. For most part we are converting RNA to DNA and sequencing the DNA just like regular DNA sequencing. - Gene model --- name: applications class: spaced # Applications - Identify gene sequences in genomes (annotation) - Learn about gene function - Differential gene expression - Explore isoform and allelic expression - Understand co-expression, pathways and networks - Gene fusion - RNA editing --- name: workflow class: spaced # Workflow .size-80[] <img src="data/rnaseq/sequence.jpg" style="height:250px; position:fixed; right:0px; bottom:0px; margin-right: 100px; margin-bottom: 130px; border-radius:4px;"> .cite[[Conesa et. al, 2016](https://genomebiology.biomedcentral.com/articles/10.1186/s13059-016-0881-8)] ??? # De-Novo assembly - When no reference genome available - To identify novel genes/transcripts/isoforms - Identify fusion genes - Assemble transcriptome from short reads - Access quality of assembly and refine - Map reads back to assembled transcriptome .tool[[Trinity](https://github.com/trinityrnaseq/trinityrnaseq/wiki)] .tool[[SOAPdenovo-Trans](http://soap.genomics.org.cn/SOAPdenovo-Trans.html)] .tool[[Oases](https://www.ebi.ac.uk/~zerbino/oases/)] .tool[[rnaSPAdes](http://cab.spbu.ru/software/rnaspades/)] .cite[[Hsieh et al, 2018](https://www.biorxiv.org/content/early/2018/08/22/380998)] .cite[[Wang et al, 2017](https://academic.oup.com/bioinformatics/article/33/3/327/2580374)] --- name: exp-design class: spaced # Experimental design .pull-left-50[ - Biological replicates: 6 - 12 .cite[[Schurch et al, 2016](http://rnajournal.cshlp.org/content/early/2016/03/30/rna.053959.115.abstract)] - Sample size estimation .cite[[Hart et al, 2013](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3842884/)] - Power analysis .cite[[RNASeqPower](https://bmcbioinformatics.biomedcentral.com/articles/10.1186/s12859-018-2191-5)] .tool[[RNASeqPower web app](https://rshiny.nbis.se/shiny-server-apps/shiny-rnaseq-power/)] - Balanced design to avoid batch effects .tool[[experDesign](https://experdesign.llrs.dev/index.html)] .tool[[DeclareDesign](https://declaredesign.org/)] - RIN values have strong effect .cite[[Romero et al, 2014](https://bmcbiol.biomedcentral.com/articles/10.1186/1741-7007-12-42)] ] .pull-right-50[ .size-90[] ] ??? - Technical replicates are not necessary .cite[[Marioni et al, 2008](https://genome.cshlp.org/content/18/9/1509.long)] - Previous publications can be useful in experimental planning to avoid repeating the same mistakes --- name: library-sequencing class: spaced # Library & Sequencing .pull-left-50[  ] .pull-right-50[  - polyA selection/Ribosomal RNA depletion - single-end/Paired-end ] ??? # Library prep - 80% of the RNA in a cell is ribosomal RNA (rRNA) - rRNA can be eliminated using polyA selection or rRNA depletion - PolyA selection mostly captures only protein-coding genes / mRNA but gives cleaner results - Depletion of rRNA is the solution if it's important to retain all RNA species - smallRNAs are purified through size selection - PCR amplification may be needed for low quantity input (See section PCR duplicates) - Preferably opt for stranded (directional) libraries .cite[[Zhao et al, 2015](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4559181/)] .cite[[Levin et al, 2010](https://www.nature.com/articles/nmeth.1491)] - Accurately identify sense/antisense transcript - Resolve overlapping genes - Exome capture - Library normalisation to concentrate specific transcripts - Libraries should have high complexity/low duplication. .cite[[Daley et al, 2013](https://www.nature.com/articles/nmeth.2375)] # Sequencing - Short reads vs long reads (Illumina/PacBio) - Read length .cite[[Chhangawala et al, 2015](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4531809/)] - Greater than 50bp does not improve DGE - Longer reads better for isoforms - Pooling samples - Sequencing depth (Coverage/Reads per sample) - Single-end reads (Cheaper) - Paired-end reads - Increased mappable reads - Increased power in assemblies - Better for structural variation and isoforms - Decreased false-positives for DGE - More replicates are better than more depth .cite[[Liu et al, 2013](https://academic.oup.com/bioinformatics/article/30/3/301/228651)] .cite[[Corley et al, 2017](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5442695/)] .cite[[SciLifeLab](https://ngisweden.scilifelab.se/file/1540-1_Comparison_of_PE_and_SE_for_RNA-seq.pdf)] --- name: workflow-dge # Workflow • DGE  --- name: read-qc # Read QC - Number of reads - Per base sequence quality - Per sequence quality score - Per base sequence content - Per sequence GC content - Per base N content - Sequence length distribution - Sequence duplication levels - Overrepresented sequences - Adapter content - Kmer content <img src="data/rnaseq/qc.jpg" style="height:250px; position:fixed; right:0px; bottom: 0px; margin-right: 100px; margin-bottom: 310px;"> .tool[[FastQC](https://www.bioinformatics.babraham.ac.uk/projects/fastqc/)] .tool[[MultiQC](http://multiqc.info/)] https://sequencing.qcfail.com/  --- name: fastqc # FastQC Good quality .size-50[[](http://www.bioinformatics.babraham.ac.uk/projects/fastqc/good_sequence_short_fastqc.html)] Poor quality .size-50[[](http://www.bioinformatics.babraham.ac.uk/projects/fastqc/bad_sequence_fastqc.html)] --- name: read-qc-2 # Read QC • PBSQ, PSQS .size-85[.small[**Per base sequence quality**] ] .size-85[.small[**Per sequence quality scores**] ] ??? # Read QC • PBSC, PSGC .size-85[.small[**Per base sequence content**] ] .size-85[.small[**Per sequence GC content**] ] # Read QC • SDL, AC .size-85[.small[**Sequence duplication level**] ] .size-85[.small[**Adapter content**] ] --- name: trimming # Trimming .pull-left-50[ - Trimming reads to remove adapter/readthrough or low quality bases - Related options are hard clipping, filtering reads - Sliding window trimming - Filter by min/max read length - Remove reads less than ~18nt - Demultiplexing/Splitting **When to avoid trimming?** - Read trimming may not always be necessary .cite[[Liao et al, 2020](https://pubmed.ncbi.nlm.nih.gov/33575617/)] - Fixed read length may sometimes be more important - Expected insert size distribution may be more important for assemblers .tool[[Cutadapt](https://github.com/marcelm/cutadapt/)] .tool[[fastp](https://github.com/OpenGene/fastp)] .tool[[Prinseq](http://prinseq.sourceforge.net/)] ] .pull-right-50[ .size-90[] ] --- name: mapping-intro # Mapping  - Aligning reads back to a reference sequence - Mapping to genome vs transcriptome - Splice-aware alignment (genome) (STAR, HISAT2 etc) .tool[[STAR](https://github.com/alexdobin/STAR)] .tool[[HiSat2](https://ccb.jhu.edu/software/hisat2/index.shtml)] .cite[[Baruzzo et al, 2017](https://www.nature.com/articles/nmeth.4106)] --- name: aligner-metrics # Aligners • Metrics .pull-left-40[  ] .pull-right-60[  .size-70[] ] .cite[[Baruzzo et al, 2017](https://www.nature.com/articles/nmeth.4106)] ??? # Aligners time and RAM |Program|Time_Min|Memory_GB| |-------|--------|---------| |HISATx1|22.7|4.3| |HISATx2|47.7|4.3| |HISAT|26.7|4.3| |STAR|25|28| |STARx2|50.5|28| |GSNAP|291.9|20.2| |TopHat2|1170|4.3| --- name: mapping-required # Mapping - Reads (FASTQ) ``` @ST-E00274:179:HHYMLALXX:8:1101:1641:1309 1:N:0:NGATGT NCATCGTGGTATTTGCACATCTTTTCTTATCAAATAAAAAGTTTAACCTACTCAGTTATGCGCATACGTTTTTTGATGGCATTTCCATAAACCGATTTTTTTTTTATGCACGTACCCAAAACGTGCAGAAAAATACGCTGCTAGAAATGTA + #AAAFAFA<-AFFJJJAFA-FFJJJJFFFAJJJJ-<FFJJJ-A-F-7--FA7F7-----FFFJFA<FFFFJ<AJ--FF-A<A-<JJ-7-7-<FF-FFFJAFFAA--A--7FJ-7----77-A--7F7)---7F-A----7)7-----7<<- ``` `@instrument:runid:flowcellid:lane:tile:xpos:ypos read:isfiltered:controlnumber:sampleid` - Reference Genome/Transcriptome (FASTA) ``` >1 dna:chromosome chromosome:GRCz10:1:1:58871917:1 REF GATCTTAAACATTTATTCCCCCTGCAAACATTTTCAATCATTACATTGTCATTTCCCCTC CAAATTAAATTTAGCCAGAGGCGCACAACATACGACCTCTAAAAAAGGTGCTGTAACATG ``` - Annotation (GTF/GFF) ``` #!genome-build GRCz10 #!genebuild-last-updated 2016-11 4 ensembl_havana gene 6732 52059 . - . gene_id "ENSDARG00000104632"; gene_version "2"; gene_name "rerg"; gene_source "ensembl_havana"; gene_biotype "protein_coding"; havana_gene "OTTDARG00000044080"; havana_gene_version "1"; ``` `seq source feature start end score strand frame attribute` .cite[[Illumina FASTQ format](https://emea.support.illumina.com/bulletins/2016/04/fastq-files-explained.html)] .cite[[GTF format](https://www.ensembl.org/info/website/upload/gff.html)] --- name: alignment # Alignment - SAM/BAM (Sequence Alignment Map format) ``` ST-E00274:188:H3JWNCCXY:4:1102:32431:49900 163 1 1 60 8S139M4S = 385 535 TATTTAGAGATCTTAAACATCCATTCCCCCTGCAAACATTTTCAATCATTACATTGTCATTTTCCCTCCAAATTAAATTTAGCCAGAGGCGCACAACATACGACCTCTAAAAAAGGTGCTGGAACATGTACCTATATGCAGCACCACCATC AAAFAFFAFFFFJ7FFFFJ<JAFA7F-<AJ7JJ<FFFJ--<FAJF<7<7FAFJ-<AFA<-JJJ-AF-AJ-FF<F--A<FF<-7777-7JA-77A---F-7AAFF-FJA--77FJ<--77)))7<JJA<J77<-------<7--))7)))7- NM:i:4 MD:Z:12T0T40C58T25 AS:i:119 XS:i:102 XA:Z:17,-53287490,4S33M4D114M,11; MQ:i:60 MC:Z:151M RG:Z:ST-E00274_188_H3JWNCCXY_4 ``` `query flag ref pos mapq cigar mrnm mpos tlen seq qual opt` **Never store alignment files in raw SAM format. Always compress it!** |Format|Size_GB| |------|-------| |SAM|7.4| |BAM|1.9| |CRAM lossless Q|1.4| |CRAM 8 bins Q|0.8| |CRAM no Q|0.26| .cite[[SAM format](http://www.htslib.org/doc/sam.html)] --- name: vis # Visualisation • IGV  .tool[[IGV](http://software.broadinstitute.org/software/igv/)] .tool[[UCSC Genome Browser](https://genome.ucsc.edu/)] .tool[[SeqMonk](https://www.bioinformatics.babraham.ac.uk/projects/seqmonk/)] ??? # Visualisation • `tview` `samtools tview alignment.bam genome.fasta`  # Visualisation • SeqMonk  .tool[[SeqMonk](https://www.bioinformatics.babraham.ac.uk/projects/seqmonk/)] --- name: alignment-qc class: spaced # Alignment QC - Number of reads mapped/unmapped/paired etc - Uniquely mapped - Insert size distribution - Coverage - Gene body coverage - Biotype counts / Chromosome counts - Counts by region: gene/intron/non-genic - Sequencing saturation - Strand specificity .tool[STAR (final log file)] .tool[samtools stats] .tool[bamtools stats] .tool[[QoRTs](https://hartleys.github.io/QoRTs/)] .tool[[RSeQC](http://rseqc.sourceforge.net/)] .tool[[Qualimap](http://qualimap.bioinfo.cipf.es/)] --- name: star-log # Alignment QC • STAR Log MultiQC can be used to summarise and plot STAR log files. .size-95[] --- name: qorts-region # Alignment QC • Features QoRTs was run on all samples and summarised using MultiQC. .size-95[] --- name: qorts-plots # Alignment QC • QoRTs .size-95[] --- name: alignment-qc-1 # Alignment QC • Examples .pull-left-50[ **Read mapping profile**  ] .pull-right-50[ **Gene body coverage**  .cite[[Sigurgeirsson et al, 2014](https://pubmed.ncbi.nlm.nih.gov/24632678/)] ] ??? The read mapping profile shows how well bases along a read mapped to the reference for all reads. Mapability usually decreases towards the 5' and 3' ends due to soft-clipping. This is is more pronounced in untrimmed reads. Gene body coverage shows read coverage over the gene for all genes. An even coverage is ideally expected. A bias in either direction could be indicative of library quality. A bias toward the 3' end is usually seen in polyA selected libraries. --- name: alignment-qc-2 # Alignment QC • Examples .pull-left-50[ **Insert size**  ] .pull-right-50[ **Saturation curve**  .cite[[Francis et al, 2013](https://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-14-167)] ] ??? Negative insert size implies overlapping mate pairs. Conserved genes in the mouse transcriptome. Saturation curves of discovery of genes in the mouse heart from a set of a subset of 248 conserved orthologs; genes which have any blast hit are shown in circles; genes which the translated protein was within the expected size range of the conserved gene are in squares; proteins which are 100% identical to a canonical protein in Uniprot/Swissprot mouse database are shown in triangles. --- name: quantification-counts class: spaced # Quantification • Counts .pull-left-50[ - Read counts = gene expression - Reads can be quantified on any feature (gene, transcript, exon etc) - Intersection on [gene models](#intro) - Gene/Transcript level  .tool[[featureCounts](http://bioinf.wehi.edu.au/featureCounts/)] .tool[[HTSeq](https://github.com/simon-anders/htseq)] ] .pull-right-50[ .size-85[.center[]] ] --- name: quant-pcr-multi # Quantification .pull-left-50[ **PCR duplicates** - Computational deduplication not recommended .cite[[Klepikova et al, 2017](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5357343/)] .cite[[Parekh et al, 2016](https://www.nature.com/articles/srep25533)] - Use PCR-free library-prep kits - Use UMIs during library-prep .cite[[Fu et al, 2018](https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-018-4933-1)] **Multi-mapping** - Added (BEDTools multicov) - Discard (featureCounts, HTSeq) - Distribute counts (Cufflinks, featureCounts) - Rescue - Probabilistic assignment (Rcount, Cufflinks) - Prioritise features (Rcount) - Probabilistic assignment with EM (RSEM) ] .pull-right-50[  ] ??? Tools that detect duplicate reads (like Picard MarkDuplicates) is looking for reads that start and end at the same coordinates. Such reads are expected to be PCR derived and can be collapsed down to 1 read. This makes sense in whole genome sequencing, but in RNA-Seq, this is naturally expected since highly expressed genes create huge number of identical transcripts which may fragment at hotspots leading to fragmentation bias .cite[[Patcher et al, 2011](https://genomebiology.biomedcentral.com/articles/10.1186/gb-2011-12-3-r22)]. Therefore, removing PCR duplicates may remove real duplicates as well. --- name: quantification-abundance # Quantification • Abundance - Count methods - Provide no inference on isoforms - Cannot accurately measure fold change <!--.size-60[]--> - Probabilistic assignment - Deconvolute ambiguous mappings - Transcript-level - cDNA reference **Kallisto, Salmon** - Ultra-fast & alignment-free - Bootstrapping & quantification confidence - Transcript-level counts - Transcript-level estimates improves gene-level estimates .cite[[Soneson et al, 2015](https://f1000research.com/articles/4-1521/v2)] .tool[[tximport](https://bioconductor.org/packages/release/bioc/html/tximport.html)] - Evaluation and comparison of isoform quantification tools .cite[[Zhang et al, 2017](https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-017-4002-1)] .tool[[RSEM](https://deweylab.github.io/RSEM/)] .tool[[Kallisto](https://pachterlab.github.io/kallisto/)] .tool[[Salmon](https://combine-lab.github.io/salmon/)] ??? Gene expression counts may be literal counts (reads overlapping to gene models) or probabilistically estimated based on the data. Proponents of probabilistically estimation claims that it is a better estimate that corrects for sampling biases. Tools such as RSEM, Kallisto and Salmon produce probabilistic abundance estimates. [Soneson et al, 2015](https://f1000research.com/articles/4-1521/v2) claims that estimating abundance using Kallisto at the transcript levels and then summarising down to gene-level counts results in improved estimates of gene expression. Kallisto/Salmon > transcript-counts > [`tximport()`](https://bioconductor.org/packages/release/bioc/html/tximport.html) > gene-counts --- name: quantification-qc # Quantification QC ``` ENSG00000000003 140 242 188 143 287 344 438 280 253 ENSG00000000005 0 0 0 0 0 0 0 0 0 ENSG00000000419 69 98 77 55 52 94 116 79 69 ENSG00000000457 56 75 104 79 157 205 183 178 153 ENSG00000000460 33 27 23 19 27 42 69 44 40 ENSG00000000938 7 38 13 17 35 76 53 37 24 ENSG00000000971 545 878 694 636 647 216 492 798 323 ENSG00000001036 79 154 74 80 128 167 220 147 72 ``` .pull-left-50[ - Pairwise correlation between samples must be high (>0.9) .size-60[] ] .pull-right-50[ - Count QC using RNASeqComp .size-80[] ] .tool[[RNASeqComp](https://bioconductor.org/packages/release/bioc/html/rnaseqcomp.html)] .cite[[Teng et al, 2016](https://genomebiology.biomedcentral.com/articles/10.1186/s13059-016-0940-1)] --- name: multiqc # MultiQC [](https://multiqc.info/examples/rna-seq/multiqc_report.html) --- name: normalisation # Normalisation - Control for Sequencing depth, compositional bias and more - Median of Ratios (DESeq2) and TMM (edgeR) perform the best  - For DGE using DGE packages, use raw counts - For clustering, heatmaps etc use VST, VOOM or RLOG - For own analysis, plots etc, use TPM - Other solutions: spike-ins/house-keeping genes <img src="data/rnaseq/distribution.jpg" style="height:240px; position:fixed; right:0px; bottom: 0px; margin-right: 70px; margin-bottom: 140px;"> .cite[[Dillies et al, 2013](https://www.ncbi.nlm.nih.gov/pubmed/22988256)] .cite[[Evans et al, 2017](https://arxiv.org/abs/1609.00959)] .cite[[Wagner et al, 2012](https://link.springer.com/article/10.1007/s12064-012-0162-3)] --- name: exploratory # Exploratory - Remove lowly expressed genes - Transform raw counts to VST, VOOM, RLOG, TPM etc - Heatmaps, MDS, PCA etc. -- .pull-left-60[  .tool[[pheatmap](https://github.com/raivokolde/pheatmap)] ] -- .pull-right-40[  ] --- name: batch-correction class: spaced # Batch correction - Estimate variation explained by variables (PVCA) .tool[[PVCA](https://bioconductor.org/packages/release/bioc/html/pvca.html)] .size-70[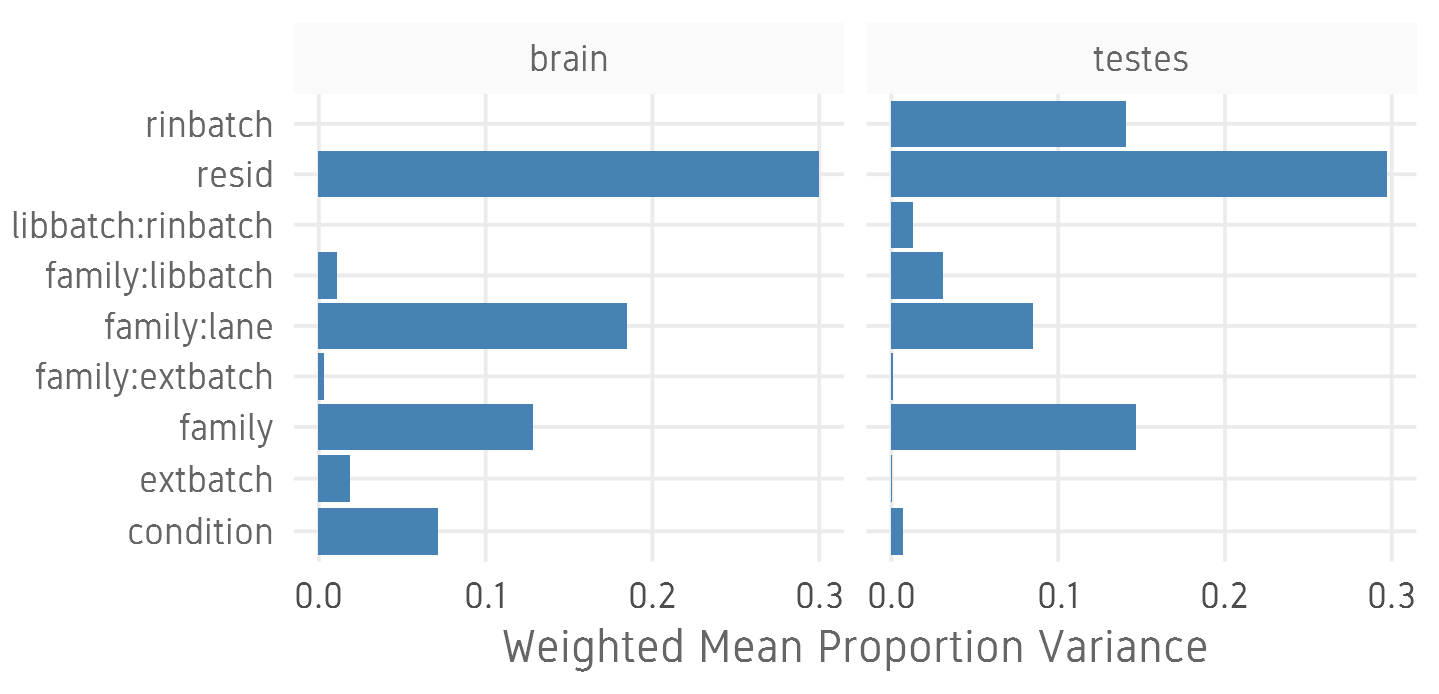] - Find confounding effects as surrogate variables (SVA) .tool[[SVA](http://bioconductor.org/packages/release/bioc/html/sva.html)] - Model known batches in the LM/GLM model - Correct known batches (ComBat from SVA)(Only if you are desperate! .cite[[Zindler et al, 2020](https://bmcbioinformatics.biomedcentral.com/articles/10.1186/s12859-020-03559-6)]) - Interactively evaluate batch effects and correction (BatchQC) .cite[[Manimaran et al, 2016](https://academic.oup.com/bioinformatics/article/32/24/3836/2525651)] .tool[[BatchQC](http://bioconductor.org/packages/release/bioc/html/BatchQC.html)] --- name: dge-1 # Differential expression .size-90[] - Univariate testing gene-by-gene - More descriptive, less predictive ??? # Differential expression - DESeq2, edgeR (Neg-binom > GLM > Test), Limma-Voom (Neg-binom > Voom-transform > LM > Test) - DESeq2 `~age+condition` - Estimate size factors `estimateSizeFactors()` - Estimate gene-wise dispersion `estimateDispersions()` - Fit curve to gene-wise dispersion estimates - Shrink gene-wise dispersion estimates - GLM fit for each gene - Wald test `nbinomWaldTest()` .size-50[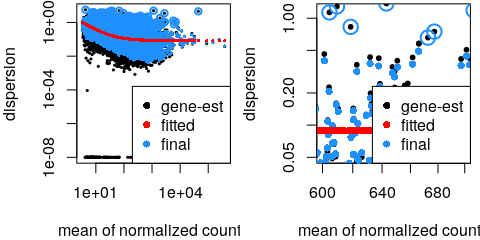] .tool[[DESeq2](https://bioconductor.org/packages/release/bioc/html/DESeq2.html)] .tool[[edgeR](https://bioconductor.org/packages/release/bioc/html/edgeR.html)] .tool[[limma](https://bioconductor.org/packages/release/bioc/html/limma.html)] .cite[[Seyednasrollah et al, 2013](https://academic.oup.com/bib/article/16/1/59/240754)] --- name: dge-3 # DGE - Results `results()` ``` log2 fold change (MLE): type type2 vs control Wald test p-value: type type2 vs control DataFrame with 1 row and 6 columns baseMean log2FoldChange lfcSE <numeric> <numeric> <numeric> ENSG00000000003 242.307796723287 -0.932926089608546 0.114285150312285 stat pvalue padj <numeric> <numeric> <numeric> ENSG00000000003 -8.16314356729037 3.26416150242775e-16 1.36240609998527e-14 ``` - Summary `summary()` ``` out of 17889 with nonzero total read count adjusted p-value < 0.1 LFC > 0 (up) : 4526, 25% LFC < 0 (down) : 5062, 28% outliers [1] : 25, 0.14% low counts [2] : 0, 0% (mean count < 3) [1] see 'cooksCutoff' argument of ?results [2] see 'independentFiltering' argument of ?results ``` --- name: dge-4 # DGE .pull-left-50[ - MA plot `plotMA()` 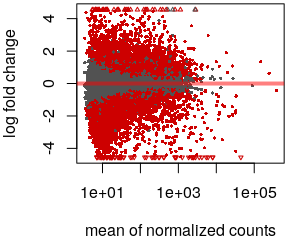 - Volcano plot  ] .pull-right-50[ - Normalised counts `plotCounts()`  <img src="data/rnaseq/scattered.gif" style="height:220px; position:fixed; right:0px; bottom:0px; margin-right: 130px; margin-bottom: 10px;"> ] --- name: functional-analysis-1 # Functional analysis • GO - Gene set analysis (GSA) - Gene set enrichment analysis (GSEA) - Gene ontology / Reactome databases .size-70[] <img src="data/rnaseq/systembio.png" style="height:220px; position:fixed; right:0px; bottom:0px; margin-right: 70px; margin-bottom: 380px;"> --- name: functional-analysis-2 # Functional analysis • Kegg - Pathway analysis (Kegg)  .cite[[DAVID](https://david.ncifcrf.gov/)] .cite[[clusterProfiler](https://bioconductor.org/packages/release/bioc/html/clusterProfiler.html)] .cite[[ClueGO](http://apps.cytoscape.org/apps/cluego)] .cite[[ErmineJ](https://erminej.msl.ubc.ca/)] .cite[[pathview](https://bioconductor.org/packages/release/bioc/html/pathview.html)] --- name: summary class: spaced # Summary - Sound experimental design to avoid confounding - Plan carefully about lib prep, sequencing etc based on experimental objective - For DGE, biological replicates may be more important than other considerations (paired-end, sequencing depth, long reads etc) - Discard low quality bases, reads, genes and samples - Verify that tools and methods align with data assumptions - Experiment with multiple pipelines and tools - QC! QC everything at every step > .large[Conesa, A., Madrigal, P., Tarazona, S., Gomez-Cabrero, D., Cervera, A., McPherson, A., ... & Mortazavi, A. (2016). A survey of best practices for RNA-seq data analysis. [Genome biology, 17(1), 1-19.](https://genomebiology.biomedcentral.com/articles/10.1186/s13059-016-0881-8)] --- name: help class: spaced # Further learning - Griffith lab [RNA-Seq using HiSat & StringTie tutorial](https://github.com/griffithlab/rnaseq_tutorial/wiki) - HBC Training [DGE using DeSeq2 tutorial](https://github.com/hbctraining/Intro-to-R-with-DGE) - Hemberg lab [scRNA-Seq tutorial](http://hemberg-lab.github.io/scRNA.seq.course/index.html) - [RNA-Seq Blog](https://www.rna-seqblog.com/) - SciLifeLab [courses](https://www.scilifelab.se/education/courses%26training) <img src="data/rnaseq/help.png" style="height:250px; position:fixed; right:0px; bottom:0px; margin-right: 35px; margin-bottom: 40px;"> --- name: end_slide class: end-slide, middle count: false # Thank you. Questions? .end-text[ <p class="smaller"> <span class="small" style="line-height: 1.2;">Graphics from </span><img src="./assets/freepik.jpg" style="max-height:20px; vertical-align:middle;"><br> Created: 26-Nov-2021 • <a href="https://www.scilifelab.se/">SciLifeLab</a> • <a href="https://nbis.se/">NBIS</a> • <a href="https://ngisweden.scilifelab.se">NGI</a> </p> ] --- name: references-1 exclude: true # References 1. Baruzzo, Giacomo, *et al*. "Simulation-based comprehensive benchmarking of RNA-seq aligners." [Nature methods 14.2 (2017): 135](https://www.nature.com/articles/nmeth.4106) 2. Busby, Michele A., *et al.* "Scotty: a web tool for designing RNA-Seq experiments to measure differential gene expression." [Bioinformatics 29.5 (2013): 656-657](https://academic.oup.com/bioinformatics/article/29/5/656/252753) 3. Conesa, Ana, *et al.* "A survey of best practices for RNA-seq data analysis." [Genome biology 17.1 (2016): 13](https://genomebiology.biomedcentral.com/articles/10.1186/s13059-016-0881-8) 4. Corley, Susan M., et al. "Differentially expressed genes from RNA-Seq and functional enrichment results are affected by the choice of single-end versus paired-end reads and stranded versus non-stranded protocols." [BMC genomics 18.1 (2017): 399](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5442695/) 5. Chhangawala, Sagar, et al. "The impact of read length on quantification of differentially expressed genes and splice junction detection." [Genome biology 16.1 (2015): 131](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4531809/) 6. Dillies, Marie-Agnes, *et al*. "A comprehensive evaluation of normalization methods for Illumina high-throughput RNA sequencing data analysis." [Briefings in bioinformatics 14.6 (2013): 671-683](https://www.ncbi.nlm.nih.gov/pubmed/22988256) 7. Evans, Ciaran, Johanna Hardin, and Daniel M. Stoebel. "Selecting between-sample RNA-Seq normalization methods from the perspective of their assumptions." [Briefings in bioinformatics (2017)](https://arxiv.org/abs/1609.00959) 8. Fu, Yu, *et al*. "Elimination of PCR duplicates in RNA-seq and small RNA-seq using unique molecular identifiers." [BMC genomics 19.1 (2018): 531](https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-018-4933-1) 9. Hart, S. N., Therneau, T. M., Zhang, Y., Poland, G. A., & Kocher, J. P. (2013). Calculating sample size estimates for RNA sequencing data. [Journal of computational biology, 20(12), 970-978](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3842884/) 10. Hsieh, Ping-Han *et al*., "Effect of de novo transcriptome assembly on transcript quantification" [2018 bioRxiv 380998](https://www.biorxiv.org/content/early/2018/08/22/380998) 11. Kim, Young-Kook, *et al*. "Short structured RNAs with low GC content are selectively lost during extraction from a small number of cells." [Molecular cell 46.6 (2012): 893-895](https://www.cell.com/molecular-cell/fulltext/S1097-2765(12)00481-9) 12. Klepikova, Anna V., *et al*. "Effect of method of deduplication on estimation of differential gene expression using RNA-seq." [PeerJ 5 (2017): e3091](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5357343/) 13. Levin, Joshua Z., et al. "Comprehensive comparative analysis of strand-specific RNA sequencing methods." [Nature methods 7.9 (2010): 709](https://www.nature.com/articles/nmeth.1491) 14. Liu, Qian, and Marianthi Markatou. "Evaluation of methods in removing batch effects on RNA-seq data." [Infectious Diseases and Translational Medicine 2.1 (2016): 3-9](http://www.tran-med.com/article/2016/2411-2917-2-1-3.html) 15. Liu, Yuwen, Jie Zhou, and Kevin P. White. "RNA-seq differential expression studies: more sequence or more replication?." [Bioinformatics 30.3 (2013): 301-304](https://academic.oup.com/bioinformatics/article/30/3/301/228651) 16. Manimaran, Solaiappan, et al. "BatchQC: interactive software for evaluating sample and batch effects in genomic data." [Bioinformatics 32.24 (2016): 3836-3838](https://academic.oup.com/bioinformatics/article/32/24/3836/2525651) 17. Marioni, John C., *et al.* "RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays." [Genome research (2008)](https://genome.cshlp.org/content/18/9/1509.long) 18. Parekh, Swati, *et al*. "The impact of amplification on differential expression analyses by RNA-seq." [Scientific reports 6 (2016): 25533](https://www.nature.com/articles/srep25533) 19. Romero, Irene Gallego, *et al*. "RNA-seq: impact of RNA degradation on transcript quantification." [BMC biology 12.1 (2014): 42](https://bmcbiol.biomedcentral.com/articles/10.1186/1741-7007-12-42) 20. Schurch, Nicholas J., *et al.* "How many biological replicates are needed in an RNA-seq experiment and which differential expression tool should you use?." [Rna (2016)](http://rnajournal.cshlp.org/content/early/2016/03/30/rna.053959.115.abstract) 21. SciLifeLab. Comparison of PE and SE for RNA-Seq, [SciLifeLab](https://ngisweden.scilifelab.se/file/1540-1_Comparison_of_PE_and_SE_for_RNA-seq.pdf) 22. Seyednasrollah, Fatemeh, *et al*. "Comparison of software packages for detecting differential expression in RNA-seq studies." [Briefings in bioinformatics 16.1 (2013): 59-70](https://academic.oup.com/bib/article/16/1/59/240754) 23. Soneson, Charlotte, *et al*. "Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences." [F1000Research 4 (2015)](https://f1000research.com/articles/4-1521/v2) 24. Teng, Mingxiang, *et al*. "A benchmark for RNA-seq quantification pipelines." [Genome biology 17.1 (2016): 74](https://genomebiology.biomedcentral.com/articles/10.1186/s13059-016-0940-1) 25. Wagner, Gunter P., Koryu Kin, and Vincent J. Lynch. "Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples." [Theory in biosciences 131.4 (2012): 281-285](https://link.springer.com/article/10.1007/s12064-012-0162-3) 26. Wang, Sufang, and Michael Gribskov. "Comprehensive evaluation of de novo transcriptome assembly programs and their effects on differential gene expression analysis." [Bioinformatics 33.3 (2017): 327-333](https://academic.oup.com/bioinformatics/article/33/3/327/2580374) 27. Zhang, Chi, *et al*. "Evaluation and comparison of computational tools for RNA-seq isoform quantification." [BMC genomics 18.1 (2017): 583](https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-017-4002-1) 28. Zhao, Shanrong, et al. "Comparison of stranded and non-stranded RNA-seq transcriptome profiling and investigation of gene overlap." [BMC genomics 16.1 (2015): 675](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4559181/) 29. Zhao, Shilin, *et al.* "RnaSeqSampleSize: real data based sample size estimation for RNA sequencing." [BMC bioinformatics 19.1 (2018): 191](https://bmcbioinformatics.biomedcentral.com/articles/10.1186/s12859-018-2191-5) --- name: lab1 class: spaced # Hands-On tutorial **Main exercise** - 01 Check the quality of the raw reads with **FastQC** - 02 Map the reads to the reference genome using **HISAT2** - 03 Assess the post-alignment quality using **QualiMap** - 04 Count the reads overlapping with genes using **featureCounts** - 05 Find differentially expressed genes using **DESeq2** in R **Bonus exercises** - 01 Functional annotation of DE genes using **GO/Reactome/Kegg** databases - 02 RNA-Seq figures and plots using **R** - 03 Visualisation of RNA-seq BAM files using **IGV** genome browser .large[**Data:** `/sw/courses/ngsintro/rnaseq/`] .large[**Work:** `/proj/snic2021-22-644/nobackup/user/rnaseq/`] --- name: lab2 # Hands-On tutorial .pull-left-40[ - Course data directory .large[`/sw/courses/ngsintro/rnaseq/`] .large[ ``` rnaseq/ +-- bonus/ | +-- assembly/ | +-- exon/ | +-- funannot/ | +-- plots/ +-- documents/ +-- main/ +-- 1_raw/ +-- 2_fastqc/ +-- 3_mapping/ +-- 4_qualimap/ +-- 5_dge/ +-- 6_multiqc/ +-- reference/ | +-- mouse_chr19_hisat2/ +-- scripts/ ``` ] ] .pull-right-60[ - Your work directory .large[`/proj/snic2021-22-644/nobackup/user/rnaseq/`] .large[ ``` [user]/ rnaseq/ +-- 1_raw/ +-- 2_fastqc/ +-- 3_mapping/ +-- 4_qualimap/ +-- 5_dge/ +-- 6_multiqc/ +-- reference/ | +-- mouse_chr19_hisat2/ +-- scripts/ +-- funannot/ +-- plots/ ``` ] ]